The field of peripheral nerve surgery has experienced significant growth over the past few years as a result of the development of more effective treatment strategies such as direct nerve coaptation or complex nerve transfers. The majority of reconstructive procedures place a high priority on restoring motor function, however sensory restoration is commonly neglected during these operations. The loss of protective sensation increases the risk of developing injuries to the body, such as corneal ulcers, pressure sores, and hand injuries on the ulnar edge. In addition, the increased risk of developing neuropathic pain or depression adversely impacts the quality of life of patients. Researchers and clinical centers have shown interest in sensory nerve reconstruction in a variety of anatomical locations. The purpose of the study is to provide a comprehensive review of the various options available for nerve transfers and direct neurotization in various parts of the body.
The clinical manifestations and consequences of peripheral nerve injuries have been well documented since ancient times. Nevertheless, the concepts of axonal repair and nerve reconstruction were not developed until the 20th century. The primary purpose of peripheral nerve surgery is to restore motor function, with sensory function being a secondary goal [1].
The loss of protective sensation increases the likelihood of injuries to the body, such as corneal ulcers, pressure wounds, or damage to the ulnar border of the hand. There is also a possibility that sensory loss may lead to autonomic dysfunctions, such as dry skin, a lack of sweating, and even impaired wound healing [2]. There is evidence that neuropathic pain and depression associated with sensory loss may have a negative effect on a patient's quality of life [3,4]. Due to the importance of regaining sensory function, the study aims to provide a comprehensive literature review of techniques developed for restoring sensory function in various parts of the body.
Over the past decade, advances in surgical techniques have enabled a wide range of strategies to be employed to restore peripheral sensory function. Among the various methods of nerve reconstruction, primary nerve repair is considered the gold standard. When there is a long gap between nerves, surgical interventions such as nerve grafting, nerve transfers, or direct neurotization may be employed [5].
Through nerve grafting and nerve transfers, sensory function is restored by stimulating the growth of axons into a native recipient nerve. End-to-end nerve repairs are the most common type of nerve repair, but end-to-side repairs allow minimal sensation loss at the donor site, which leads to collateral sprouting in sensory nerves without an epineurial window [5,6].
If nerve endings cannot be repaired, direct neurotization may be the last option. The technique involves the connection of nerve fascicles to a target organ such as the cornea or skin. While the exact mechanism of action is unknown, it may also restore sensation and trophism [7].
In contrast to motor reconstruction, sensory reconstruction appears not to be time-sensitive, and there is no consensus regarding a maximum timeframe [6]. The following sections provide an overview of reconstructive procedures performed on the face, the breast, the upper extremities, and the lower body. Table 1 summarizes various nerve options that can be utilized in reconstructive surgery, depending on the area of the body being treated.
Trigeminal Sensory Neuropathy
The trigeminal nerve transmits sensory information from the head and neck through its ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions. There are several etiologies that may result in changes in facial sensation, such as fractures, intracranial tumors, and iatrogenic injuries [8]. With trigeminal anesthesia, there is a risk of injury to the soft tissues of the face [9]. Injuries commonly observed during trigeminal anesthesia are biting of the lip, cheek, and tongue on the affected side, which may occur without nociceptive feedback. Occasionally, trigeminal anesthesia can result in corneal ulceration, which can eventually lead to blindness in some cases. Trigeminal trophic syndrome can also develop in a subset of patients following trigeminal anesthesia [9].
Tactile sensation can be restored in the maxillary (V2) and mandibular (V3) territories by transferring axons from the contralateral infraorbital and mental nerves either directly or by means of cross-face grafts. Several studies have demonstrated that facial sensation can be restored within a few months following surgery. Kaban and Upton report the case of a female patient who gradually regained sensation of the left mental nerve distribution as well as light touch and protective sensations one year after surgery [10]. Koshima et al. reported two cases of trigeminal nerve II palsy that were repaired by contralateral trigeminal nerve transfer without the use of any nerve graft [11]. The affected upper labial sensory recovery was 1.65 to 2.44 (Semmes-Weinstein values) and 15 to 30 mm (moving 2-point discriminations) in the 12 to 18 months following surgery. Catapano et al. published a case series in which the return of protective sensation was obtained after 5 months of surgery [12]. Moreover, tactile sensation in the lower lips was restored after 12 months of treatment. A case reported by Mucci and Dellon described a successful transfer of the supraclavicular nerve to the mental nerve, which resulted in the return of protective sensation and tactile sensation to the lower lip [13].
Neurotrophic Keratopathy
It is well known that neurotrophic keratopathy greatly increases the risk of corneal ulcers, scarring, and loss of vision. A potentially curative surgical procedure, corneal neurotization, has recently been introduced as a means of preventing complications associated with neurotrophic keratopathy for patients. It has been shown that this procedure can effectively restore corneal sensitivity and reverse corneal changes [14].
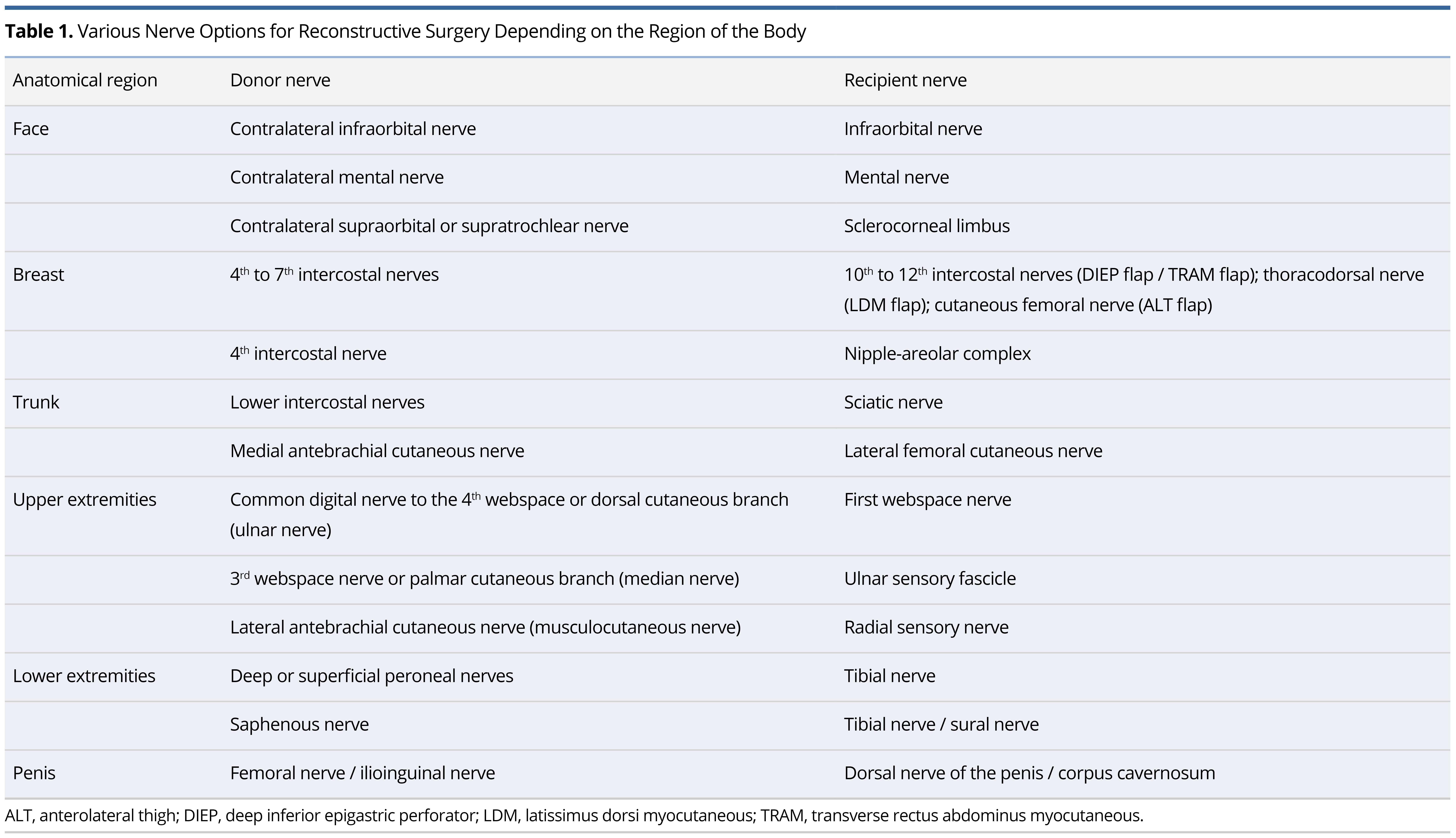
As early as 2009, Terzis et al. reported direct corneal neurotization through the transfer of contralateral supraorbital and supratrochlear nerves to the sclerocorneal limbus (direct corneal neurotization, Figure 1) [14]. The technique was later simplified by interposing nerve grafts between the contralateral supratrochlear nerve and the cornea (indirect corneal neurotization) [15]. Both direct and indirect approaches to corneal neurotization result in comparable improvements in corneal sensitivity, trophism, and sub-basal nerve restoration after surgery [16].
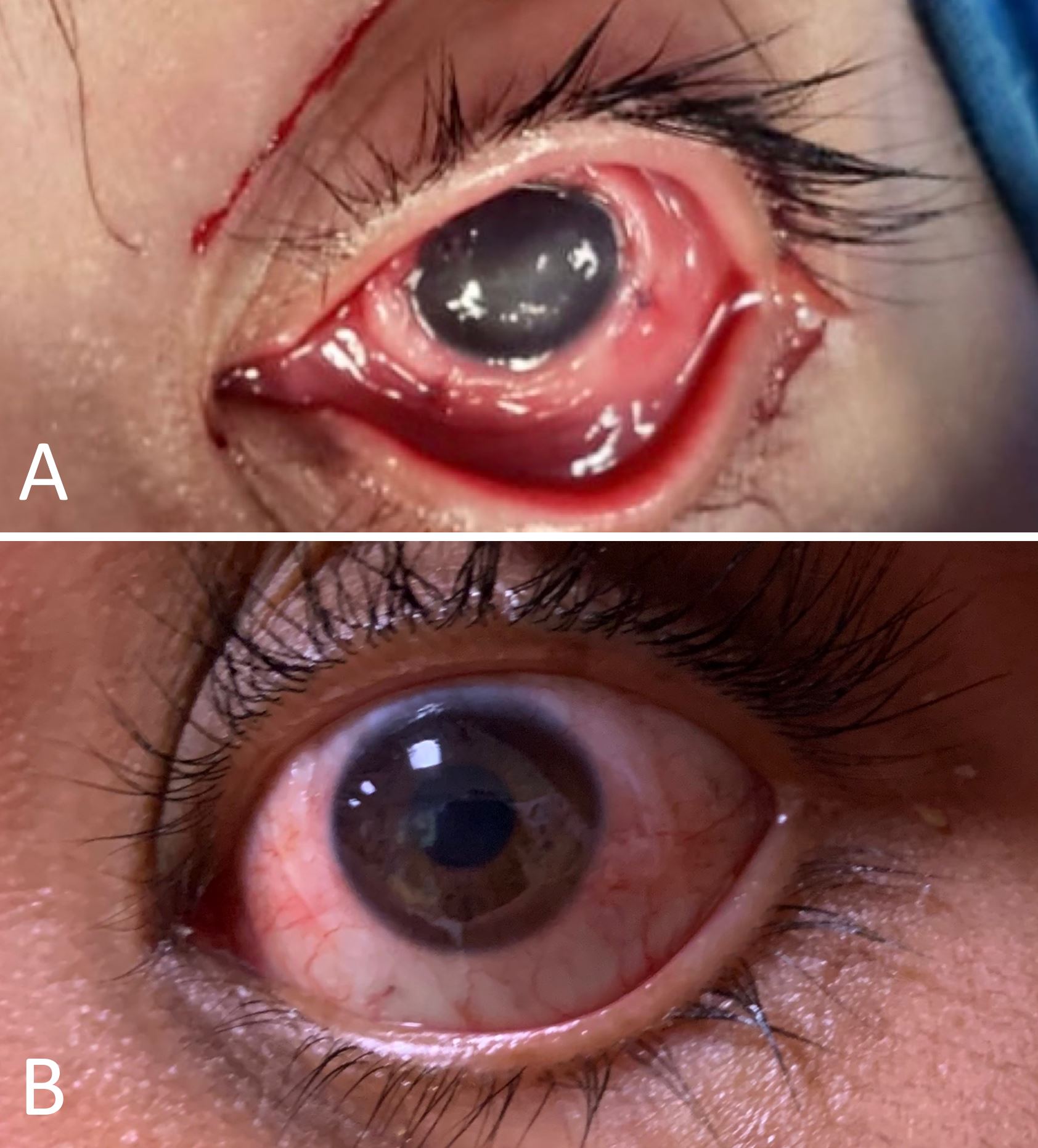
Figure 1. The case illustrates a 16-year-old male with neurotrophic keratopathy following the removal of an intracranial tumor. (A) A photograph taken during surgery demonstrates the opacity of the cornea and the nerve graft ends at the sclerocorneal limbus. (B) The postoperative photograph shows that the opacity has resolved one year following the procedure.
Lingual Nerve Neuropathy
The lingual nerve is particularly susceptible to iatrogenic damage during the extraction of the third molar due to the proximity of the nerves in the area, resulting in pain, altered sensations, and even numbness in the anterior third of the tongue [17]. It has been demonstrated that indirect graft nerve repair, whether using an autograft or an allograft, is associated with improved subjective and objective nerve outcomes when compared to direct nerve repair [17]. However, in special circumstances such as large oncological resections where the ipsilateral trunk is not available, bridging from the contralateral lingual nerve may be necessary [2].
The technique of oncological breast surgery has evolved towards less invasive techniques with improved aesthetics. Nevertheless, diminished breast sensation after a mastectomy remains one of the most common complaints. There is a possibility that spontaneous sensory recovery may occur, but the timing of recovery is unpredictable. According to the literature, the rate of recovery may vary between 0% and 47% [18-20].
Harvesting a sensory cutaneous nerve from the transferred flap is an option for providing sensation following autologous breast reconstruction. A variety of different nerves are commonly used as donor nerves. The 10th and 12th intercostal nerves are commonly used as donor nerves for abdominal flaps, the thoracodorsal nerve for the latissimus dorsi flap, and the lateral femoral cutaneous nerve for the anterolateral thigh flap. These nerves are usually coapted to the 4th to 7th intercostal nerves at the recipient site [21,22].
There is evidence from two systematic reviews that the sensory function of neurotized flaps may recover more quickly than that of non-neurotized flaps, which may result in improved quality of life for patients [18,23]. Research has shown that abdominal-based flaps and latissimus dorsi flaps provide the most favorable results. While neurotization has produced positive results, its effectiveness is still subject to debate due to its added operating time, donor site morbidity, and cost [23].
Recent studies have suggested that it may be possible to direct neurotize the nipple-areola complex by using the 4th intercostal nerve after nipple-preserving mastectomy. There have been several promising early reports on the recovery of sensation 10 to 15 months after the procedure [24-26].
Hand and digital nerve reconstruction are commonly performed procedures to restore fingertip sensation, which is essential for fine motor tasks [27]. In most cases, direct nerve repair or nerve graft interposition results in successful results; however, distal nerve transfers are required in patients with large nerve gaps or proximal injuries [28-30].
Median Nerve Deficit
The median nerve deficit results in loss of sensation along the volar aspects of the thumb, index, long, and radial borders of the ring fingers. In order to restore sensory function to the first web space, terminal branches of the ulnar nerve (such as the common digital nerve) can be transferred to the fourth web space or the dorsal cutaneous branch to the first web space nerves in a direct end-to-end manner. It has been reported that the common digital nerves of the second and third web spaces can be transferred end-to-side to the ulnar digital nerve of the small finger to restore protective sensation in less critical distributions [30].
Ulnar Nerve Deficit
The ulnar nerve injury results in diminished sensation in the fourth web space and on the ulnar side of the small finger, which are essential for grasping and protective sensation. Reconstruction can be accomplished by performing an end-to-end transfer from the median nerve fascicle to the third web space and to the ulnar sensory fascicle. In this area, alternative donors may include the palmar cutaneous nerve branch or even the median nerve proper [31]. When both the median and ulnar nerves are injured, it may be beneficial to transfer the superficial branch of the radial nerve to both targets to restore protective sensation to their respective territories [32,33].
Radial Nerve Deficit
The dorsum of the hand is not considered to be a critical surface, however, some strategies have been developed to restore sensory function in patients with radial nerve injuries, such as a direct end-to-side transfer of the radial nerve to the median nerve, or a lateral antebrachial cutaneous nerve transfer to the radial nerve [28].
Sensate reconstruction of the lower body is of significant benefit to patients who have suffered spinal cord injuries or peripheral nerve damage in their lower limbs. The loss of protective sensation can result in repeated trauma, chronic non-healing ulcers, infections, malnutrition, and even amputation of the limbs if it is not treated in time [34].
Transfer of Sensory Branches from the Peroneal Nerve
Several nerve transfers have been described for the legs, although these are far less common than those described for the upper extremities. It was initially reported by Gordon and Buncke [35] that nerve transfers from the sensory branches of the peroneal nerve to either the tibial nerve or the sural nerve can restore protective sensation to the plantar region [35-37].
Transfer of the Saphenous Branch of the Femoral Nerve
In cases where there is no access to the peroneal nerves, such as when proximal sciatic nerve injuries have occurred, it may be possible to transfer the saphenous branch of the femoral nerve to the distal tibial nerve or sural nerve. The method has been shown to be capable of recovering sensitivity up to useful discriminatory sensitivity in accordance with the British Medical Research Council classification in two case studies [38,39].
Pressure Ulcers
The incidence of pressure ulcers among patients with spinal cord injuries ranges from 10 to 20% [34]. Typical management strategies include continuous mobilization of the patient as well as regional flap rotation to eliminate dead spaces. Unfortunately, insensate flaps may also result in ulceration over time.
Although several motor nerve transfers have been described for tetraplegic patients, only two types of sensory nerve transfers have been reported to prevent paraplegic pressure ulcers in patients by improving sacral sensation or providing sensory feedback to pressure-bearing areas. According to Mackinnon et al. [40], the medial antebrachial cutaneous nerve can be transferred to the lateral femoral cutaneous nerve. In one study, Viterbo and Ripari performed nerve transfers from the intercostal nerve to the sciatic nerve [41].
In the 1970's, Dibbell [42] and Daniel et al. [43] described a technique for mobilizing sensate tissue over an anesthetic area using long flaps innervated by the intercostal nerves. It should be noted that in subsequent studies several regional sensate flaps were described, with the ideal flap often depending on the level of injury. As one example, the tensor fascial lata flap can be performed if the lesion is below the level of the L3/4 [44], and the sensate pedicled anterolateral thigh flap can be performed if the lesion is below the level of the L2 [45].
Anterolateral Thigh Flaps
With the advancement of microsurgery, it is now possible to create sensate free flaps using sensory nerves that are available in the lower limbs or above the level of spinal cord damage [46]. Anterolateral thigh flaps that are neurotized by the lateral femoral cutaneous nerve have been the most extensively studied flaps, with reported superior aesthetic results and the possibility of early rehabilitation and function. In the study, 100% of the subjects had regained touch and nociception, and 85% had regained thermal sensitivity (Figure 2) [47]. Comparison between neurotized and non-neurotized flaps found that 76% of patients had sensory recovery after 3 months in the neurotized group, compared to 20% in the non-neurotized group [48].
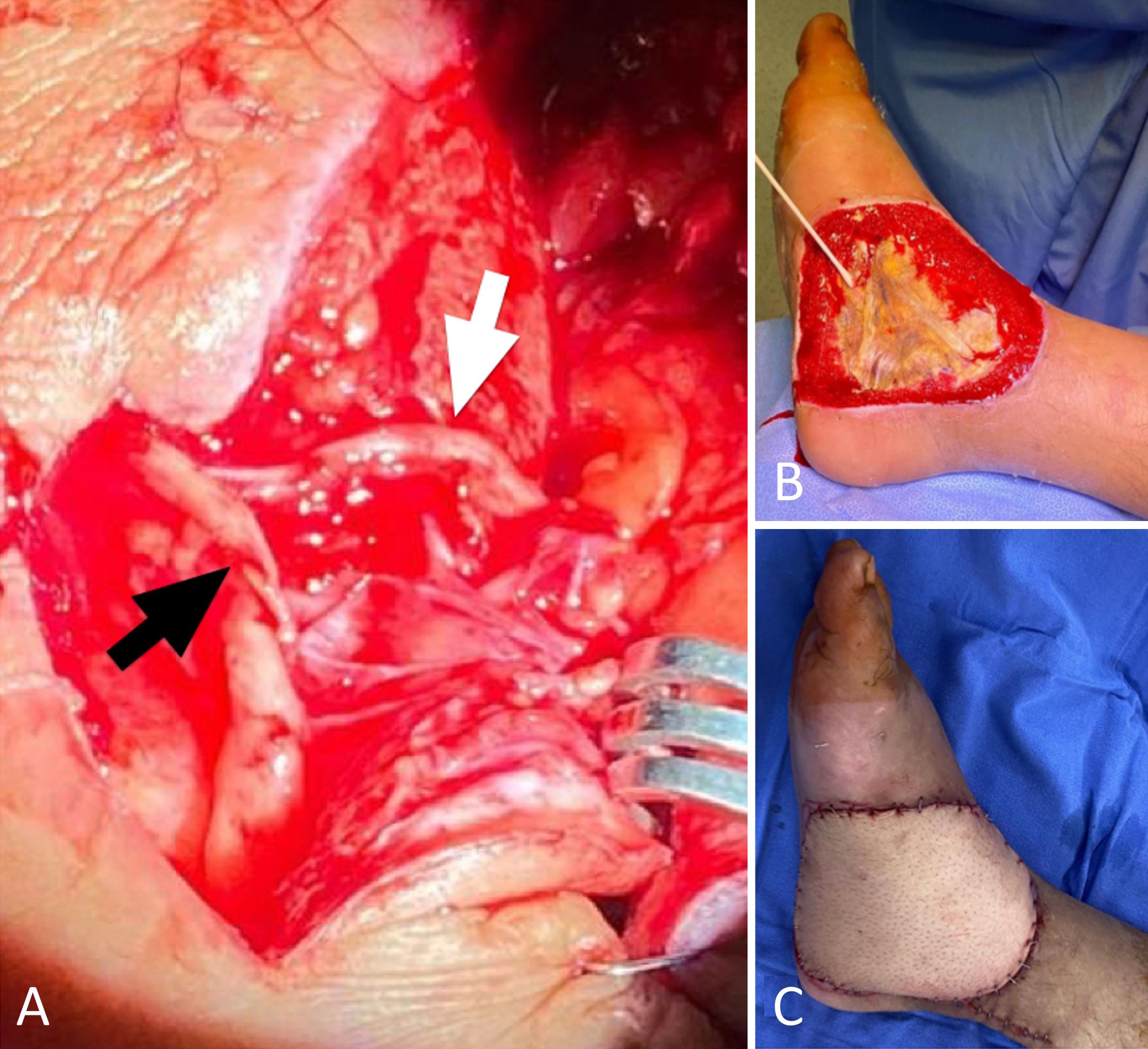
Figure 2. This case illustrates the neurotized anterolateral thigh flap performed on a 50-year-old male with third degree burns on his lower leg and foot. (A) This intraoperative photography illustrates the end-to-side neurorrhaphy from the femoral cutaneous nerve (white arrow) to the superficial peroneal nerve (black arrow). (B) A photograph taken prior to surgery. (C) The postoperative outcome.
Erectile dysfunction is a common complication after a radical prostatectomy. Currently, the majority of management strategies are based on pharmacological interventions. In some cases, surgeons have attempted direct repair or grafting of the pudendal nerve, but the results have been unsatisfactory [49,50]. Souza et al. reported in 2017 that the femoral nerve may be used to neurotize the dorsal nerve of the penis and the corpus cavernosum [50]. Thirteen months after reinnervation surgery, 60% of patients were able to achieve full penetration. According to their findings, penile reinnervation surgery proved to be a viable and effective method for treating erectile dysfunction following radical prostatectomy.
In patients with low spinal injuries and normal inguinal sensations, it is possible to improve sensation, sexuality, and quality of life through the TOMAX (TO MAX-imize sensation, sexuality, and quality of life) procedure, which consists of neurotizing the dorsal nerve of the penis with the ilioinguinal nerve. According to its proponents, 24 of 30 patients (80%) reported a return to glans sensation after receiving the TOMAX procedure [51].
There is evidence that phalloplasty may be complemented by flap neurotization. This can either be done on the dorsal nerve of the penis in males, or on the inguinal nerve in transgender individuals. A complete return to sensation was observed in both groups [52,53].
In recent studies, it has been suggested that females who have suffered genital mutilation may benefit from the reconstruction of the sensate clitoris through the use of neurotized flaps attached to the pudendal nerve [54] and sensate labial flaps [55], although the results from such procedures are currently limited.
Although many advances have been made in the field of sensory nerve transfers and direct neurotization, it appears that specialized rehabilitation protocols capable of facilitating the reconstruction of sensory functions will be the direction of this field in the future. A second concern is the difficulty of objectively evaluating postoperative outcomes due to the wide range of parameters that can be measured, including tactile sensation, temperature, vibration, and erogenous sensation.
Sensory nerve reconstruction is one of the most fascinating, innovative, and pioneering chapters in peripheral nerve reconstruction. Numerous surgical techniques have been described in the literature; however, evidence-based results are lacking, as most of the reports are retrospective case studies, which are likely to be biased. A surgeon should be aware, however, that sensory restoration is an achievable goal during peripheral nerve reconstruction and should be explored as a potential means of improving the patient's quality of life.
Received date: March 29, 2022
Accepted date: September 13, 2022
Published date: November 30, 2022
The authors would like to thank Dr. Tommy Nai-Jen Chang for his valuable input and for taking the time to share his insights with them. Additionally, the authors wish to acknowledge the support provided by the International Microsurgery Journal and the International Microsurgery Club.
The manuscript has not been presented at any meetings on the topic.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The authors obtained permission from the participants in the human research prior to publishing their images or photographs.
This research has received no specific grant from any funding agency either in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest declared by either the authors or the contributors of this article, which is their intellectual property.
It should be noted that the opinions and statements expressed in this article are those of the respective author(s) and are not to be regarded as factual statements. These opinions and statements may not represent the views of their affiliated organizations, the publishing house, the editors, or any other reviewers since these are the sole opinion and statement of the author(s). The publisher does not guarantee or endorse any of the statements that are made by the manufacturer of any product discussed in this article, or any statements that are made by the author(s) in relation to the mentioned product.
© 2022 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY). In accordance with accepted academic practice, anyone may use, distribute, or reproduce this material, so long as the original author(s), the copyright holder(s), and the original publication of this journal are credited, and this publication is cited as the original. To the extent permitted by these terms and conditions of license, this material may not be compiled, distributed, or reproduced in any manner that is inconsistent with those terms and conditions.
Video 1. Conversations with SciTeMed editors and expert mentors.
The communication among international microsurgeons have switched from one direction (from paper, textbook) to multiway interactions through the internet. The authors believe the online platform will play an immensely important role in the learning and development in the field of microsurgery.
Combining intraoperative ICG lymphography with NIR vein visualization can aid supermicrosurgeons in identifying lymphatic vessels and superficial venules to guide LVA incision placement. This guided approach significantly improves successful creation of LVAs when compared to the blind (anatomic) approach. In addition, the absence of linear ICG lymphographic patterns does not prevent formation of successful LVAs.
Immediate limb compression following the LVA procedure facilitates lymphatic drainage and increases the surgical efficacy by increasing the number of functioning anastomoses, and is a recommended postoperative practice.
Traditionally, suturing techniques have been the mainstay for microvascular anastomoses, but owing to its technical difficulty and labour intensity, considerable work has gone into the development of sutureless microvascular anastomoses. In this review, the authors take a brief look at the developments of this technology through the years, with a focus on the more recent developments of laser-assisted vascular anastomoses, the unilink system, vascular closure staples, tissue adhesives, and magnets. Their working principles, with what has been found concerning their advantages and disadvantages are discussed.
This case report demonstrates an important supermicrosurgical technique for lymphedema, which was established by Isao Koshima in 1994. So far, over 2,000 cases of limb edema have been treated by this surgical method.
The authors proposed a new less invasive island flap, namely the first metatarsal artery capillary perforator flap. The advantages of this flap include the preservation of the first metatarsal artery and the adiposal tissue in the web space, thereby preventing compression around the remaining deep peroneal nerve.
Prof. Koushima, president of World Society for Reconstructive Microsurgery, proposes an innovative concept and technique of the multi-stage ‘Orochi’ combined flaps (sequential flaps in parallel). The technique opens a new vista in reconstructive microsurgery.
This study reviews the reconstruction modalities within the multidisciplinary approach for chest wall reconstruction. The authors have proposed an algorithm that is formulated based on their experience to manage chest wall defects following cancer resection.
LVA and vascularized lymph node transfer VLNT are established lymphedema treatments. However, LVA is only effective for early disease and VLNT can cause donor-site lymphedema and contour deformity. VLVT is free of these limitations. The authors described their experience of a new VLVT technique.
The video presents a useful technique for microvascular anastomosis in reconstructive surgery of the head and neck. It is advantageous to use this series of sutures when working with limited space, weak vessels (vessels irradiated, or with atheroclastic plaques), suturing in tension, or suturing smaller vessels (less than 0.8 cm in diameter).
Authors discuss a silicone tube that provides structural support to vessels throughout the entire precarious suturing process. This modification of the conventional microvascular anastomosis technique may facilitate initial skill acquisition using the rat model.
PEDs can be used as alternative means of magnification in microsurgery training considering that they are superior to surgical loupes in magnification, FOV and WD ranges, allowing greater operational versatility in microsurgical maneuvers, its behavior being closer to that of surgical microscopes in some optical characteristics. These devices have a lower cost than microscopes and some brands of surgical loupes, greater accessibility in the market and innovation plasticity through technological and physical applications and accessories with respect to classical magnification devices. Although PEDs own advanced technological features such as high-quality cameras and electronic loupes applications to improve the visualizations, it is important to continue the development of better technological applications and accessories for microsurgical practice, and additionally, it is important to produce evidence of its application at surgery room.
Avulsion injuries and replantation of the upper arm are particularly challenging in the field of traumatic microsurgery. At present, the functional recovery of the avulsion injuries upper arm after the replantation is generally not ideal enough, and there is no guideline for the surgeries. The aim of this study was to analyze the causes of failure of the upper arm replantation for avulsion injuries, summarize the upper arm replantation’s indications, and improve the replantation methods.
Osteoarthritic finger joints are often repaired with joint implants, arthrodesis, or a vascularized interphalangeal joint graft. However, grafts can damage the donor toe. Based on the results of this study, the authors suggest that vascularized distal interphalangeal joint transfers from the second toe may be suitable for reconstructing these defects through microsurgery.
The supraclavicular flap has gained popularity in recent years as a reliable and easily harvested flap with occasional anatomical variations in the course of the pedicle. The study shows how the determination of the dominant pedicle may be aided with indocyanine green angiography. Additionally, the authors demonstrate how they convert a supraclavicular flap to a free flap if the dominant pedicle is unfavorable to a pedicled flap design.
The implications of rebound heparin hypercoagulability following cessation of therapy in microsurgery is unreported. In this article the authors report two cases of late digit circulatory compromise shortly after withdrawal of heparin therapy. The authors also propose potential consideration for changes in perioperative anticoagulation practice to reduce this risk.
In a cost-effective and portable way, a novel method was developed to assist trainees in spinal surgery to gain and develop microsurgery skills, which will increase self-confidence. Residents at a spine surgery center were assessed before and after training on the effectiveness of a simulation training model. The participants who used the training model completed the exercise in less than 22 minutes, but none could do it in less than 30 minutes previously. The research team created a comprehensive model to train junior surgeons advanced spine microsurgery skills. The article contains valuable information for readers.
The loupe plays a critical role in the microsurgeon's arsenal, helping to provide intricate details. In the absence of adequate subcutaneous fat, the prismatic lens of the spectacle model may exert enormous pressure on the delicate skin of the nasal bone. By developing a soft nasal support, the author has incorporated the principle of offloading into an elegant, simple yet brilliant innovation. A simple procedure such as this could prove invaluable for microsurgeons who suffer from nasal discoloration or pain as a result of prolonged use of prismatic loupes. With this technique, 42% of the pressure applied to the nose is reduced.
An examination of plastic surgery residents' experiences with microsurgery in Latin American countries was conducted in a cross-sectional study with 129 microsurgeons. The project also identifies ways to increase the number of trained microsurgeons in the region. The authors claim that there are few resident plastic surgeons in Latin America who are capable of attaining the level of experience necessary to function as independent microsurgeons. It is believed that international microsurgical fellowships would be an effective strategy for improving the situation.
This retrospective study on the keystone design perforator island flap (KDPIF) reconstruction offers valuable insights and compelling reasons for readers to engage with the article. By sharing clinical experience and reporting outcomes, the study provides evidence of the efficacy and safety profile of KDPIF as a reconstructive technique for soft tissue defects. The findings highlight the versatility, simplicity, and favorable outcomes associated with KDPIF, making it an essential read for plastic surgeons and researchers in the field. Surgeons worldwide have shown substantial interest in KDPIF, and this study contributes to the expanding knowledge base, reinforcing its clinical significance. Moreover, the study's comprehensive analysis of various parameters, including flap survival rate, complications, donor site morbidity, and scar assessment, enhances the understanding of the procedure's outcomes and potential benefits. The insights garnered from this research not only validate the widespread adoption of KDPIF but also provide valuable guidance for optimizing soft tissue reconstruction in diverse clinical scenarios. For readers seeking to explore innovative reconstructive techniques and improve patient outcomes, this article offers valuable knowledge and practical insights.
This comprehensive review article presents a profound exploration of critical facets within the realm of microsurgery, challenging existing paradigms. Through meticulous examination, the authors illuminate the intricate world of microangiosomes, dissection planes, and the clinical relevance of anatomical structures. Central to this discourse is an exhaustive comparative analysis of dermal plexus flaps, meticulously dissecting the viability and potential grafting applications of subdermal versus deep-dermal plexi. Augmenting this intellectual voyage are detailed illustrations, guiding readers through the intricate microanatomy underlying skin and adjacent tissues. This synthesis of knowledge not only redefines existing microsurgical principles but also opens new frontiers. By unearthing novel perspectives on microangiosomes and dissection planes and by offering a comparative insight into dermal plexus flaps, this work reshapes the landscape of microsurgery. These elucidations, coupled with visual aids, equip practitioners with invaluable insights for practical integration, promising to propel the field of microsurgery to unprecedented heights.
This article presents a groundbreaking surgical approach for treating facial paralysis, focusing on the combination of the pronator quadratus muscle (PQM) and the radial forearm flap (RFF). It addresses the challenges in restoring facial functions and skin closure in paralysis cases. The study's novelty lies in its detailed examination of the PQM's vascular anatomy when combined with the RFF, a topic previously unexplored. Through meticulous dissections, it provides crucial anatomical insights essential for enhancing facial reanimation surgeries, offering significant benefits in medical practices related to facial reconstruction and nerve transfer techniques.
This article exemplifies a significant advancement in microsurgical techniques, highlighting the integration of robotic-assisted surgery into the deep inferior epigastric perforator (DIEP) flap procedure for breast reconstruction. It demonstrates how innovative robotic technology refines traditional methods, reducing the invasiveness of surgeries and potentially lessening postoperative complications like pain and herniation by minimizing the length of the fascial incision. This manuscript is pivotal for professionals in the medical field, especially those specializing in plastic surgery, as it provides a comprehensive overview of the operative techniques, benefits, and critical insights into successful implementation. Moreover, it underscores the importance of ongoing research and adaptation in surgical practices to enhance patient outcomes. The article serves as a must-read, not only for its immediate clinical implications but also for its role in setting the stage for future innovations in robotic-assisted microsurgery.
The groundbreaking study illuminates the complex mechanisms of nerve regeneration within fasciocutaneous flaps through meticulous neurohistological evaluation, setting a new benchmark in experimental microsurgery. It challenges existing paradigms by demonstrating the transformative potential of sensory neurorrhaphy in animal models, suggesting possible clinical applications. The data reveal a dynamic interplay of nerve recovery and degeneration, offering critical insights that could revolutionize trauma management and reconstructive techniques. By bridging experimental findings with hypothetical clinical scenarios, this article inspires continued innovation and research, aimed at enhancing the efficacy of flap surgeries in restoring function and sensation, thus profoundly impacting future therapeutic strategies.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
Authors present a case of combined proximal median and ulnar nerves extensive injury that was successfully managed using a novel safe strategy implemented.
This study introduces an advanced tubularized radial artery forearm flap (RAFF) technique, marking an enhancement over traditional methods in addressing complex nasal reconstructions. It integrates functional and aesthetic considerations through a structured, multi-stage reconstruction process, emphasizing the use of tubularized flaps. Key learning points include the detailed crafting of stable nasal passages, strategic use of costal cartilage for robust structural support, and tailored postoperative care with silicone splints. The tubularized RAFF technique not only optimizes patient outcomes and quality of life but also provides plastic surgeons with critical insights to refine their techniques in facial reconstruction. Indispensable for professionals in the field, this article enriches the understanding of sophisticated reconstructive challenges and solutions.
This article presents a crucial case report on potential wound healing complications linked to fremanezumab, a calcitonin gene-related peptide-targeting antibody for migraine prevention. It documents the first known instance of delayed wound healing following a free flap breast reconstruction, underscoring the need for heightened clinical vigilance and individualized patient assessment in perioperative settings. Highlighting significant safety data gaps, the report advocates for comprehensive research and rigorous post-marketing surveillance. The findings emphasize the importance of balancing the risks of delayed wound healing with the need for effective disease control, especially when using biologic agents for chronic conditions. This article is essential for medical professionals managing patients on biologic therapies, offering critical insights and advocating for a personalized approach to optimize patient outcomes. By presenting novel observations and calling for further investigation, it serves as a vital resource for enhancing patient care and safety standards in the context of biologic treatments and surgical interventions.
This manuscript showcases an advanced surgical approach for treating malignant giant cell tumor of bone, emphasizing precision and ethical considerations. It leverages innovative pedicled flap technologies, as opposed to free flaps, enhancing limb functionality and patient quality of life. This technique equips surgeons with evidence that tailored surgical strategies can significantly improve outcomes in complex cases. The paper discusses technical challenges and highlights the application of supercharging and superdrainage techniques in limb reconstructions, methods well-established in microsurgery but infrequently used in oncological contexts. These techniques are crucial for optimizing flap viability and ensuring surgical success. Additionally, the manuscript underscores the profound impact of these advancements on patient lives, offering hope and showcasing tangible benefits. This narrative, blending scientific analysis with patient stories, enriches the understanding of limb reconstruction innovations in oncological surgery, making it invaluable for surgeons.
This article presents the first comprehensive review of refractory chylous ascites associated with systemic lupus erythematosus, analyzing 19 cases to propose an evidence-based therapeutic framework. It introduces lymphatic bypass surgery as an effective option for this rare complication, overcoming the limitations of conventional treatment. By integrating mechanical drainage, immunomodulation, and lymphangiogenesis, this approach achieves rapid and sustained resolution of ascites. The findings offer a novel surgical strategy for autoimmune lymphatic disorders and prompt a re-evaluation of their complex pathophysiology. This study demonstrates how surgical innovation can succeed where traditional therapies fail, offering new hope in managing refractory autoimmune disease.
Motorcycle chain-induced fingertip amputations represent a reconstructive dead end, where severe crushing and contamination traditionally compel revision amputation. The authors dismantle this exclusion criterion, reporting an 83% salvage rate using a modified protocol of radical debridement, strategic skeletal shortening, and simplified single-vessel supermicrosurgery. By eschewing complex grafting for tension-free primary anastomosis, the authors successfully restored perfusion in ostensibly
The corresponding author, Dr. Jose E. Telich-Tarriba, met with the editors and expert mentors of SciTeMed to maximize the impact and dissemination of the article. We were delighted to have five distinguished guests participate in this webinar, including Dr. Ronald M. Zuker, Dr. Sami Tuffaha, Dr. Peter Neligan, Dr. Hari Venkatramani, and Dr. Tommy Nai-Jen Chang. Dr. Tommy Nai-Jen Chang is the Editor-in-Chief of the International Microsurgery Journal. Dr. Ronald M. Zuker and Dr. Peter Neligan both serve as Honorary Editors-in-Chief of the International Microsurgery Journal. The webinar was moderated by Dr. Urska Cebron, a medical doctor and researcher at the HELIOS Klinikum Emil von Behring in Berlin, Germany.
Ronald M. Zuker, MD, FRCSC, FACS, FAAP, FRCSEd (Hon)

Dr. Ronald M. Zuker is one of the most prominent pioneers in the field of microsurgery. His publications include more than 100 peer-reviewed scientific papers and book chapters. A notable achievement of his career has been the publication of Principles and Practice of Pediatric Plastic Surgery (co-edited with Bruce Bauer and Mike Bentz), which is the standard text for pediatric plastic and reconstructive surgery. Dr. Zuker is best known for his work as the "Smile Doctor" since he has dedicated himself to restoring function to patients suffering from facial paralysis and Moebius syndrome. He has revolutionized the approach to the treatment of established facial paralysis through the use of free functioning muscle transfers, in which the gracilis muscle plays a critical role. The experience of Dr. Zuker in separating conjoined twins (seven) is one of the largest in the world. Through the implementation of microsurgery into the liver transplant team, Dr. Zuker has set the bar high and created unprecedented success rates following living donor liver transplants. In addition, he performed the first lower limb allotransplant in the world. There is an anatomic point named after him. In June 2014, Dr. Zuker was awarded the Canadian Society Lifetime Achievement Award for his contributions to the field of Plastic & Reconstructive Surgery.
Sami Tuffaha, MD

Dr. Sami Tuffaha is a hand surgeon with fellowship training at The Curtis National Hand Center. He is also an Assistant Professor in the Departments of Plastic Surgery, Orthopedic Surgery, and Neurosurgery at Johns Hopkins University. Dr. Tuffaha completed his residency at the Johns Hopkins/University of Maryland Plastic and Reconstructive Training Program. Following this, he completed a fellowship in Hand and Upper Extremity Surgery at the Mayo Clinic, where he focused on microsurgery and peripheral nerve surgery. Dr. Tuffaha has a special interest and expertise in treating peripheral nerve disorders. His research program integrates basic, translational, and clinical research in order to develop strategies to enhance nerve regeneration and functional recovery following peripheral nerve injury. Among these efforts is an ongoing clinical trial examining the efficacy of a therapeutic agent he developed in the lab.
Peter Neligan, MB, FACS, FRCSI, FRCSC

Dr. Peter Neligan is the Honorary Editor-in-Chief of the International Microsurgery Journal. He is a former president of the Plastic Surgery Foundation, of the American Society for Reconstructive Microsurgery, and of the North American Skull Base Society. He is also a former board member of the American Head & Neck Society. Previously, he served as a trustee of the American Society of Plastic Surgeons. Dr. Neligan has authored more than 12 books, 85 book chapters, and over 200 peer-reviewed publications. At present, he serves as Editor-in-Chief of Plastic Surgery, a six-volume textbook used throughout the world for plastic surgery training. He has been invited to over 300 universities and major societies as a visiting professor or honored guest. Besides serving on several editorial boards, he has previously served as Editor-in-Chief of the Journal of Reconstructive Microsurgery.
Hari Venkatramani, MD

With over 21 years of experience as a plastic and reconstructive surgeon, Dr. Hari Venkatramani is an expert in his field. He specializes in reconstructive surgery, including the reconstruction of major limbs and the reconstruction of the brachial plexus. He also performs microsurgery for the reconstruction of cancer and the treatment of lymphedema. Currently, he is a senior consultant at the Department of Plastic, Hand, and Reconstructive Microsurgery at the prestigious Ganga Hospital in Coimbatore, which is one of the largest trauma, plastic surgery, and orthopedic hospitals in India. Dr. Venkatramani serves as secretary of the Indian Society for Reconstructive Microsurgery as well as the Brachial Plexus Surgery Group of India. During his career, he has published 75 peer-reviewed journal articles as well as 12 book chapters.
Tommy Nai-Jen Chang, MD

Dr. Tommy Nai-Jen Chang is a board-certified plastic surgeon in Taiwan. He is an Associate Professor in the Division of Reconstructive Microsurgery, Department of Plastic and Reconstructive Microsurgery, Chang-Gung Memorial Hospital, Linkou Medical Center, Taiwan. In his current position, Dr. Chang is collaborating with Professor David Chwei-Chin Chuang in the area of peripheral nerve reconstruction for brachial plexus injuries in adults and children, facial paralysis, upper and lower extremity reconstruction, as well as decompression neuropathy, including carpal tunnel syndrome, ulnar tunnel syndrome, thoracic outlet syndrome and neurogenic tumors. A total of 80 articles have been published in the literature until the year 2022, mainly in the field of reconstructive microsurgery. Among his research interests are peripheral nerve regeneration and reconstruction, as well as the use of social media in microsurgery education. Dr. Chang founded the International Microsurgery Club in 2016; until now, this group represents the largest online microsurgery platform. Dr. Chang was appointed editor-in-chief of the International Microsurgery Journal in 2017, and in 2018, he founded the International Microsurgery Website to provide educational resources for microsurgery surgeons worldwide.
Telich Tarriba JE, Alvarez G, Cardenas-Mejia A. Sensory nerve transfers and direct neurotization: The new frontier in peripheral nerve surgery. Int Microsurg J 2022;6(2):2. https://doi.org/10.24983/scitemed.imj.2022.00167