Objective: We prescribe the tibial osseo-periosteal (TOP) flap for complex nonunions and bone defects, which we believe is thinner, larger, and more accessible to elevate and to cover larger areas compared to other popular existing options. This addition increases the number of donor sites for vascularized osseo-periosteal flaps.
Methods: From 1992 to 1997, we operated upon seven patients; four males and three females in the age range of 25 to 71 years. TOP flaps were based on the proximal perforator of the posterior tibial artery and encompassed five islanded and two free periosteal flaps.
Results: A 100% flap survival rate was achieved with different permutations and combinations. There were no serious complications. The prime indication was bony nonunions in a non- or poorly vascularized bed. Depending on the nature of the defect, the required skin cover and/or the volume augmentation was provided as chimeric components, pro ne rata. All cases exhibited optimal bone healing. The advantages of this flap are its relatively large size, decreased donor site morbidity, minimal anatomical variation and flexibility of chimeric flap options either as vascularized cancellous bone grafts and/or adjacent skin paddles. However, the application has a technical limitation since it requires super-microsurgical expertise.
Conclusion: The TOP flap is minimally invasive with significant advantages regarding the morbidity and complications of other osseo-periosteal flaps, thereby making it a possible application for non-unions and bone defects.
Vascularized bone grafts have been used for a wide variety of bone defects and non-unions, especially for those in ischemic zones. Although osseous non-unions have been reconstructed with free non-vascularized bone grafts, the best outcomes have been with vascularized bone grafts. The reconstruction methods for bony non-unions initially involved using the larger flaps such as the deep circumflex iliac artery perforator flap and the fibular osseous flap. However, these were associated with significant donor site morbidities, such as meralgia paraesthetica in the former option.
Doi et al. introduced a free vascularized cortico-periosteal flap based on the descending genicular artery and this helped in expanding the use of vascularized bone grafts in specific clinical situations such as pseudoarthrosis of the carpal bone defects [1-6]. Nevertheless, its use has been limited due to its small size and anatomical position. While postoperative morbidity of medial femoral condyle flap was less than its previous osseous flap alternatives, traversing the deeper layers of the thigh still entails some morbidity and minimizes the possibility of harvesting a thin chimeric skin paddle required for the resurfacing of hand or facial defects.
To further improve the usage of osseo-periosteal flaps, the senior author (Isao Koshima) envisaged an osseo-periosteal flap. Using this flap, surgeons may be able to harvest either cancellous or cortical bone as well as a thinner chimeric skin paddle, which is an ideal option for covering extremity and facial defects. This idea led to the innovative development of the tibial osseo-periosteal (TOP) flap. TOP flap is a larger periosteal flap than the medial femoral condyle. In addition, TOP flap has a favorable anatomical position with a thin skin around it, hence it is a good option for an island flap. It is based on a single proximal perforator of the posterior tibial artery. In this article, we describe our experience of the first reported cohort of TOP flaps in clinical practice.
Seven patients with extremity and facial defects were treated with five islanded and two free TOP flaps in a retrospective case series over five years from 1992 to 1997. For management of the bony non-unions and complex defects, all islanded variants were performed for the lower limb defects while free chimeric TOP flaps were applied to the hand and orbito-maxillary regions. The cohort included four males and three females in the age range of 25 to 71 years (mean age of 50 years). The underlying etiology varied from burns wounds and bony infection to radiotherapy-induced bony non-unions.
Anatomy
Prior to embarking on clinical cases, cadaveric dissection was repeatedly performed to identify and delineate the relevant anatomy. Cutaneous perforators were first located from the proximal one-third of the lower leg to the medial malleolus through the medial side of the tibia before being traced proximally to their sources [7-9]. Three types of perforators were identified: septocutaneous, musculocutaneous, and periosteal. The first group passes from the posterior tibial artery through the intermuscular septum between the soleus/calcaneal tendon and the flexor digitorum longus muscle before penetrating the fascia to arborize into the overlying skin. Muscle perforators, on the other hand, pierce and on most occasions perfuse the muscle bellies in the proximal half of the leg before irrigating the skin [7]. The periosteal perforators traverse the septal space between the medial border of the tibia and the soleus muscle, and were noted to perfuse both the underlying periosteum as well as the overlying skin (Figure 1). These perforators are confined to the proximal third of the leg while the septocutaneous perforators were mainly located in the distal third of the leg.
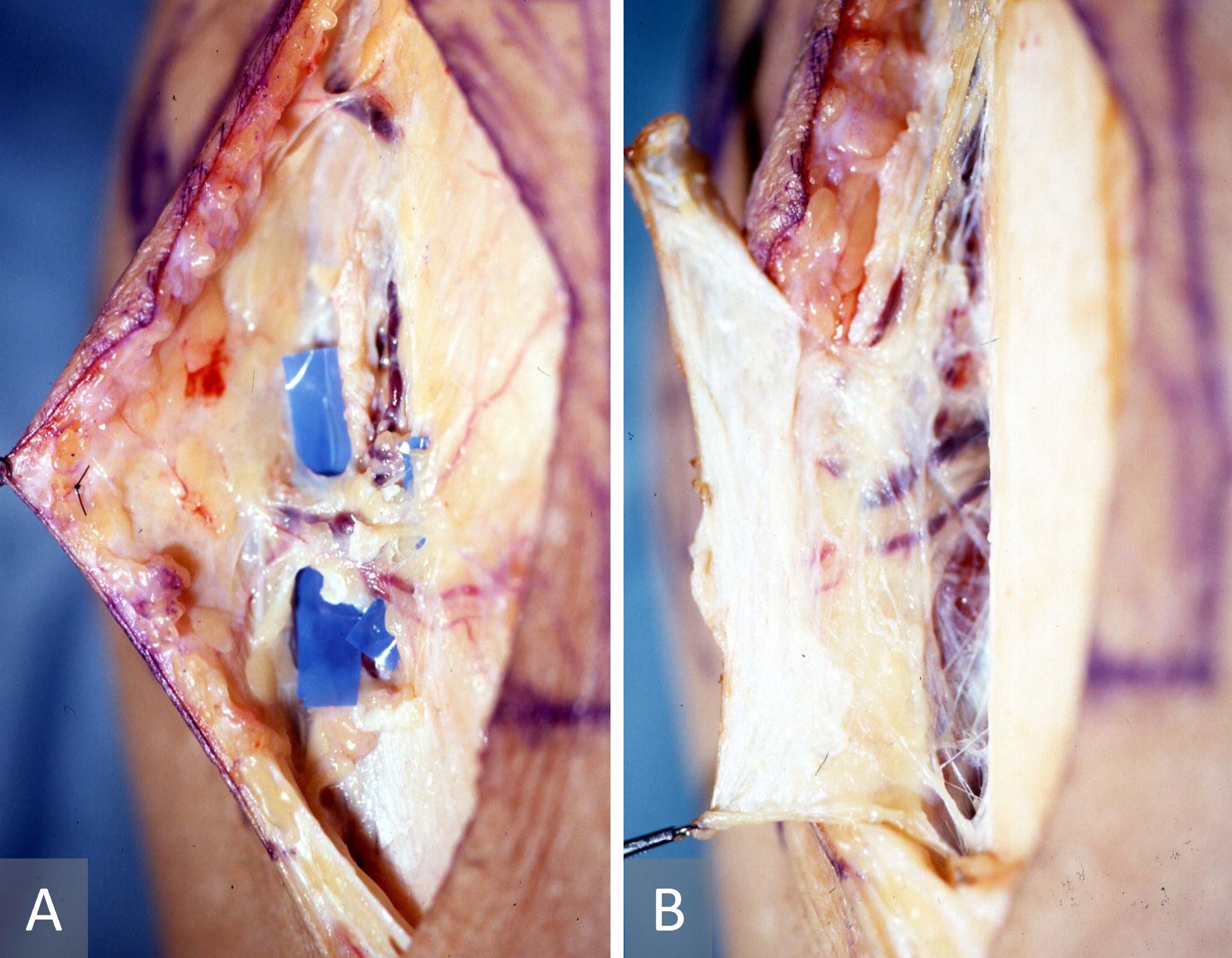
Figure 1. Anatomy of the proximal perforator from the posterior tibial artery which ramifies superficially as a cutaneous perforator (A) and delves deeper as a cortico-periosteal perforator (B).
Surgical Technique
The position of the penetrating perforator branch was examined with a hand-held Doppler device (Huntleigh Ltd., UK) at the medial margin of the tibia in the proximal one third of the lower leg [7-9]. A 5-cm incision was made along the medial aspect on the tibia centering on the signal point and the posterior/medial skin was elevated from the incision. It was necessary to confirm that the perforator of the posterior tibial artery travelled along the septal septum between the medial tibial margin and the soleus muscle. Normally, this penetrating branch was divided into nutrient vessels of the tibial periosteum and the anterior skin of the tibia. After this confirmation, the tibial periosteum was adequately exposed. The perforator was also confirmed before designing the osseo-periosteal flap. After a periosteal incision was made, the periosteal flap was elevated from the lateral to the medial to reach a periosteal perforator as a pedicle. Further dissection was performed if a monitor cutaneous flap or a volume-filling adipose flap was required, proximally along the two flap component pedicles until the common perforator was identified. Once dissection passed deeper to the muscle plane, it could reach the posterior tibial artery and the concomitant veins. It should be noted that in the case of an island flap, the deeper dissection to the source vessel was unnecessary. In the case of a free flap, however, transection should be done at the level of origin of the perforator from the posterior tibial artery (Figure 2).
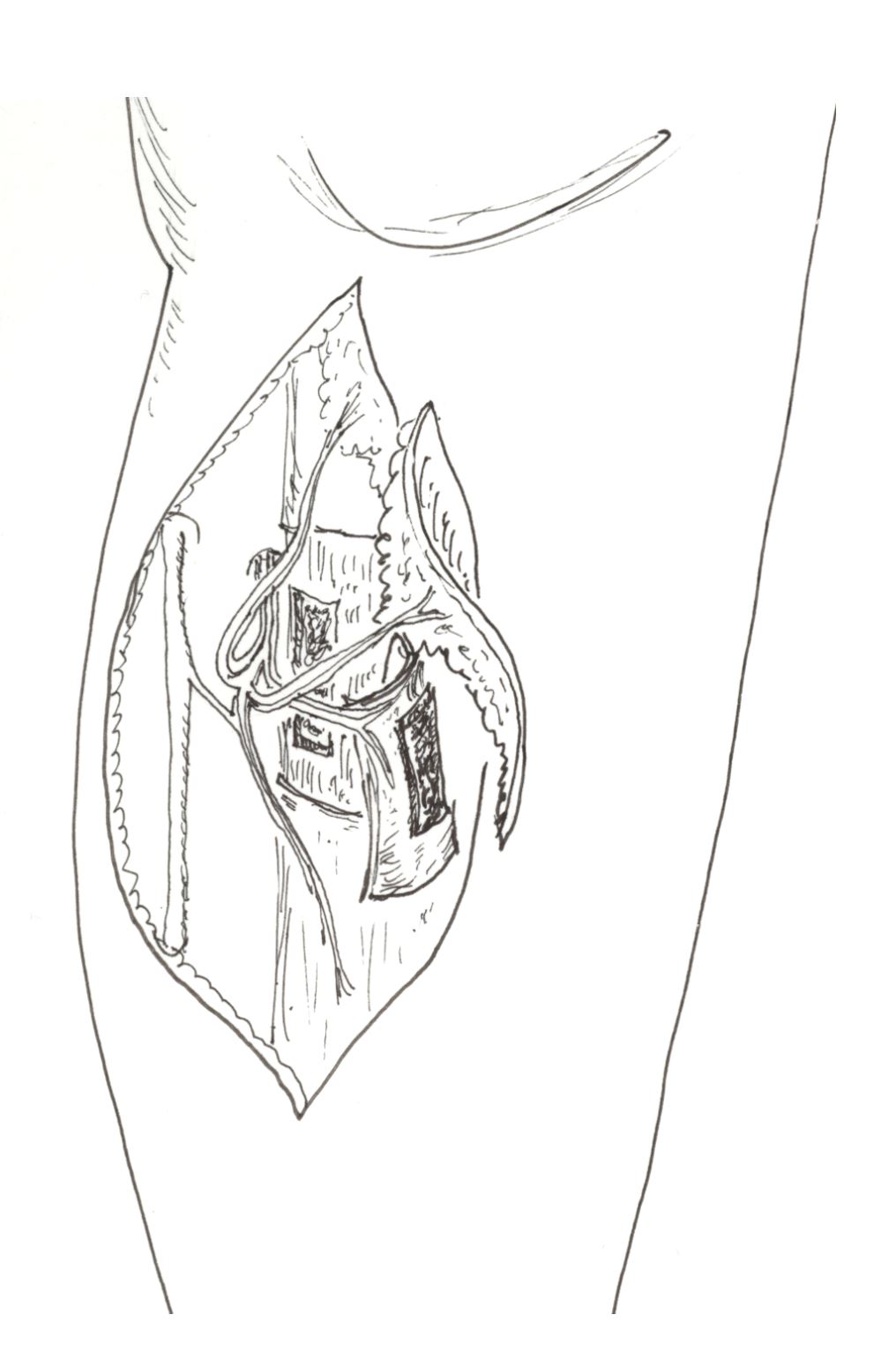
Figure 2. Graphical illustration of the chimeric tibial osseo-periosteal flap with its associated skin paddle.
Table 1 shows the patient characteristics. There was a 100% flap survival rate in all seven cases with no documented significant postoperative complications. Bony union was observed in four cases, and function recovery was noted in all cases. Complex defects were successfully treated in terms of contour match. Specific details for select cases are discussed below.
Case 1
This case describes an islanded periosteo-cutaneous flap harvested with a previous meshed skin graft. A 42-year-old man had sustained burns to both his lower legs four months previously and had been treated by meshed skin grafting with a harvested islanded periosteo-cutaneous flap (Figure 3). A recalcitrant non-healing wound was observed with exposed tibial bone over the anterior aspect of the right lower leg. To cover this defect, an islanded periosteo-cutaneous flap including the previous meshed skin graft was elevated with a perforator from the posterior tibial vessel. The flap was outlined on the grafted skin over the medial aspect of the tibia and was then transferred as a pedicled flap to cover the exposed tibia. The donor defect was reconstructed with a split skin graft. The patient recovered fully. Post-operative recovery was uneventful with complete flap survival and a healed wound.
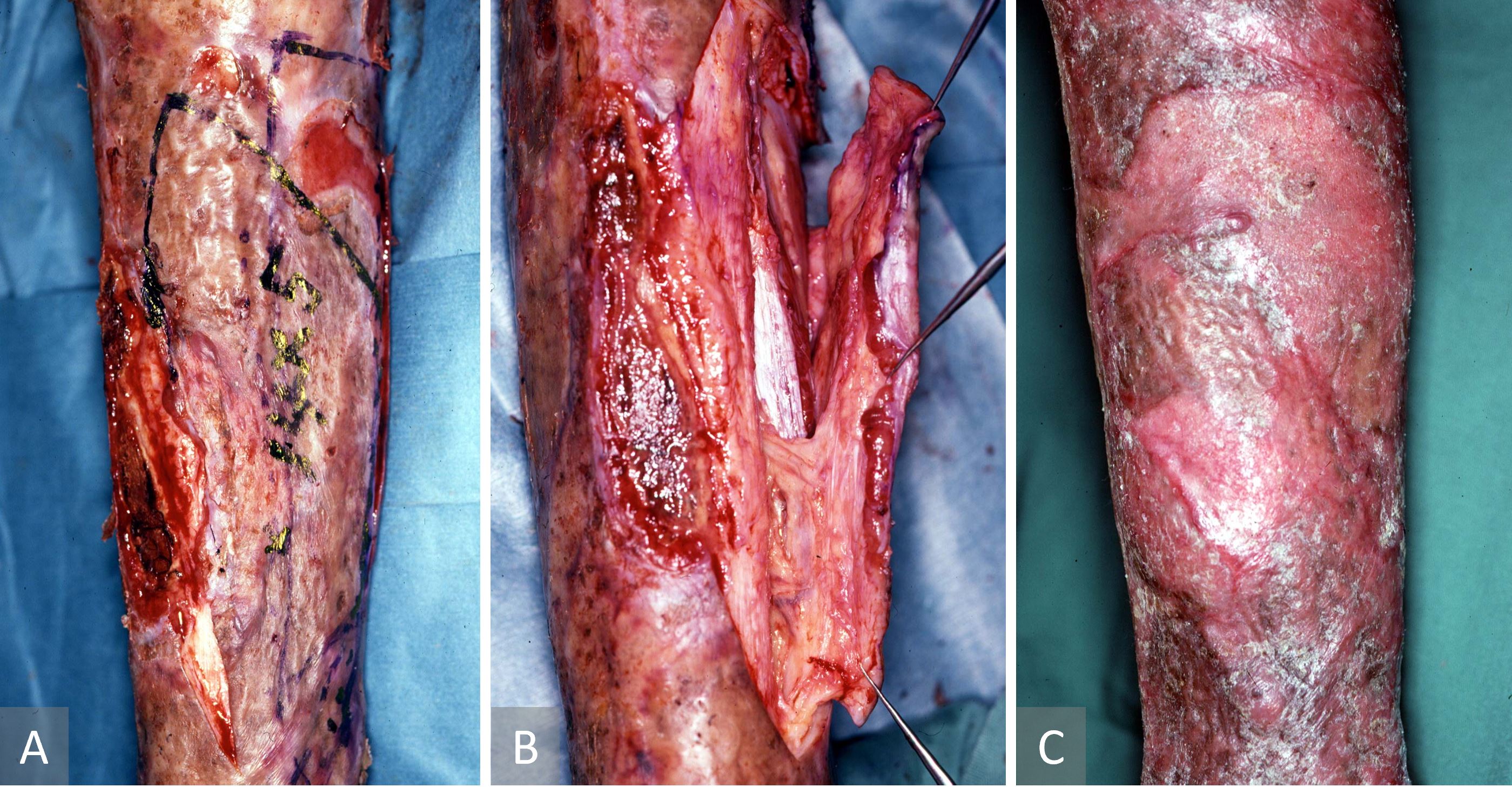
Figure 3. Case 1 describing an islanded periosteo-cutaneous flap harvested with a previous meshed skin graft. (A) Preoperative pre-tibial ulcer. (B) Islanded tibial osseo-periosteal flap raised with the overlying meshed skin graft. (C) Healed leg four months after surgery.
Case 2
This case describes a supercharged islanded osseo-periosteal flap for bony nonunion. A 55-year-old-woman suffered from a tibiofibular open fracture following a road traffic accident six months earlier. The case was complicated with a subsequent Methicillin-resistant Staphylococcus aureus infection. Radiographic evidence of the tibial non-union was also observed (Figure 4A). A supercharged islanded osseo-periosteal flap was used for bony non-union. Despite repeated surgical exploration and necrotic bone debridement followed by external fixation, there was a tibial non-union and a non-healing wound over the distal third of the tibia. The tibial bone defect was packed with cancellous iliac bone graft and a bipedicled vascularized TOP flap. The middle perforator was preserved, and the proximal perforator was transected before pivoting the flap through 180 degree and anastomosing it to a distal leg perforator (supercharged propeller flap, Figure 4B-C). The bone graft was wrapped by the TOP flap to maximize both osseo-induction and osseo-conduction. Postoperatively, the unstable ulcer healed without the need for a skin graft or a local flap. There was no infection or absorption of the grafted bone. Two months after surgery, radiographic evidence of complete resolution of the tibial non-union was observed following the application of the supercharged tibial osseo-periosteal flap propeller flap (Figure 4D). The patient was able to walk with crutches.
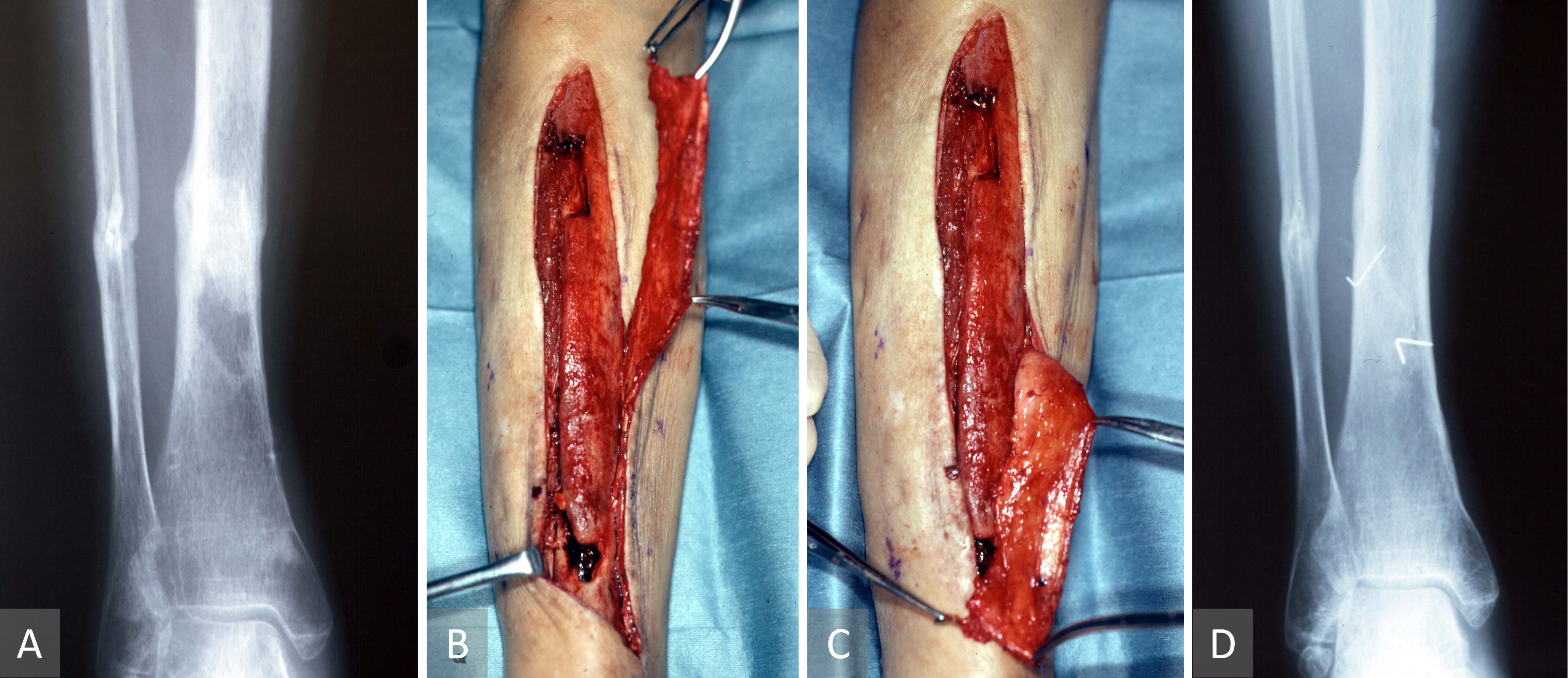
Figure 4. Case 2 describing a supercharged islanded osseo-periosteal flap for bony nonunion. (A) Radiographic evidence of the tibial non-union. (B, C) Propeller tibial osseo-periosteal flap pivoted 180 on its middle perforator with the proximal perforator anastomosed to a distal leg perforator as a supercharging maneuver. (D) Post-operative radiographic evidence of complete resolution of the tibial non-union following the application of the supercharged tibial osseo-periosteal flap propeller flap.
Case 3
This case describes a free chimeric TOP flap with a skin paddle for bony non-union (Figure 5). A 24-year-old male suffered from an avulsion amputation of left thumb, which had not been replanted. Five months later, a wrap-around free flap with an iliac bone graft had been performed for thumb reconstruction. However, non-union occurred with bone absorption at the middle portion of the grafted bone. Ten months after the thumb reconstruction, we performed a surgery in which a free vascularized chimeric TOP flap was raised with a skin paddle and anastomosed to the radial digital artery of the index finger. The radial digital artery was transected at the level of proximal inter-phalangeal joint of the index finger and was transposed to the thumb to serve as the recipient artery. It was noted intra-operatively that the posterior tibial perforator had two branches: a cutaneous branch and a periosteal branch. Two concomitant veins entered the perforating vein and long saphenous vein. The perforators were transected at the level of muscle fascia (vessel diameter at 0.6 mm). The bone defect was packed with cancellous bone graft from the tibia bone marrow, and the surface was covered with the tibial cortex, which was fixed by mini-screws and C-wires. The grafted tibial cortex was then wrapped within the TOP flap, which was in turn anastomosed to the transferred digital artery of the index finger and the dorsal cutaneous vein of the thumb. The skin defect was closed with the monitoring skin flap. The donor defect on the pretibial region was closed.
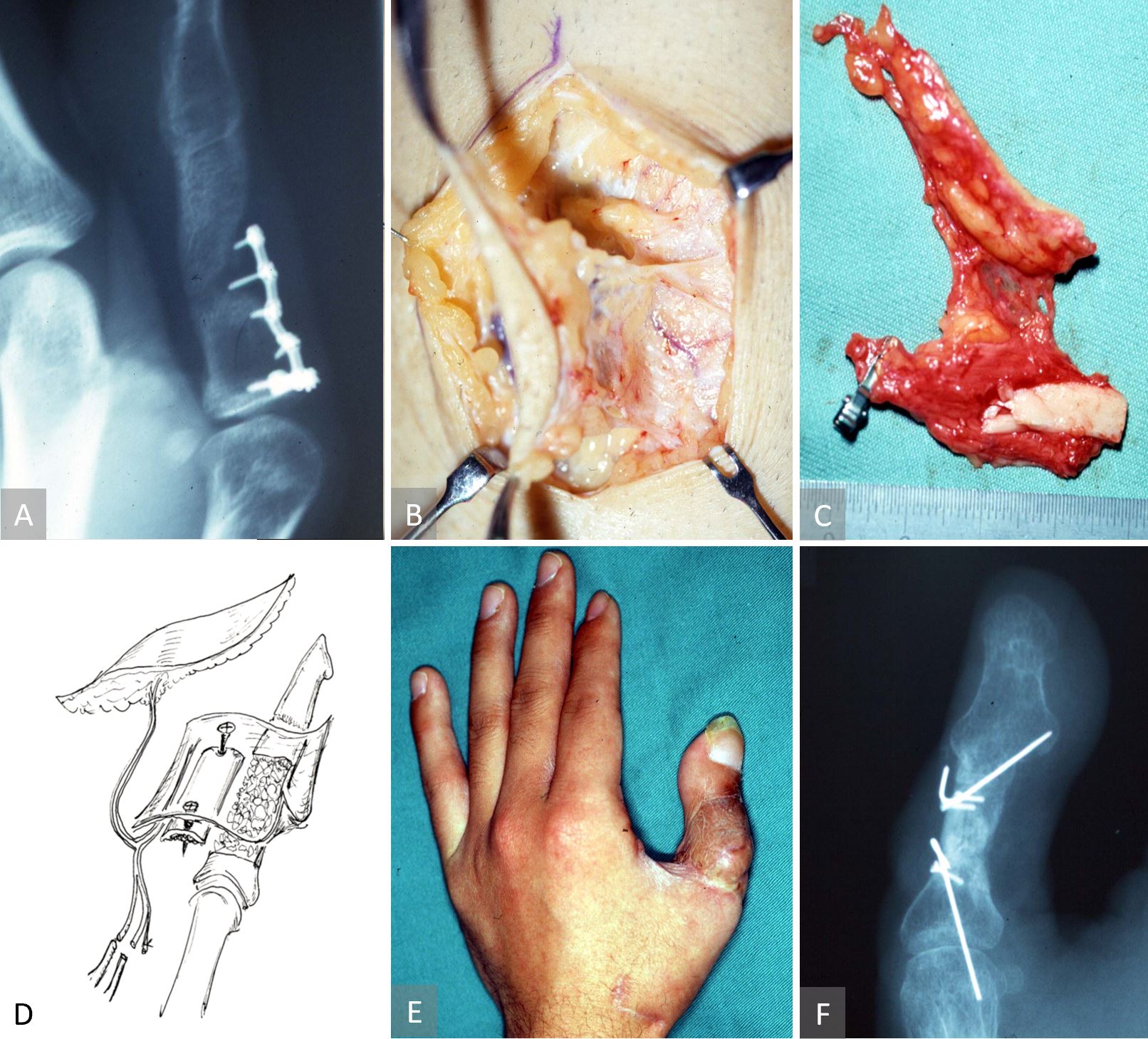
Figure 5. Case 3 describing a free chimeric TOP flap with a skin paddle for bony non-union. (A) Radiographic evidence of a non-united iliac crest bone graft. Elevation of the chimeric tibial osseo-periosteal flap with a skin paddle (B) and its orientation as a free flap (C). An artist’s impression of the chimeric tibial osseo-periosteal flap with its cortico-periosteal component being used to reconstruct the non-healed osseous defect. The skin paddle is used to resurface the composite defect as well as acting as a monitoring component of the chimeric flap (D). Postoperative image of a few months later, showing a viable chimeric tibial osseo-periosteal flap (E) with radiographic evidence of bony union (F).
Case 4
This case illustrates a sequential TOP and anterolateral thigh free flaps for the reconstruction of an orbito-maxillary defect. A 55-year-old male patient had developed an aggressive maxillary carcinoma. He had undergone chemotherapy and radiation therapy before an en-bloc resection of the left maxilla and orbital floor. The defect was left unreconstructed in order to detect recurrence. After five years, no evidence of tumor recurrence was noted, and he was referred for reconstruction. A free composite adipo-periosteal TOP flap was made to rebuild the infraorbital fat pad and orbital floor. To augment the deep cheek fat pads and to achieve a symmetrical postoperative malar contour, an anterolateral thigh flap was also raised and anastomosed to the perforator vessels of the TOP flap at one end and anastomosed to the facial vessels at its proximal end.
Vascularized bone grafts remain the gold standard for reconstruction in cases where both osseoinduction and osseoconduction are necessary, as in cases of bony non-union. As mentioned earlier, large flaps such as the deep circumflex iliac artery perforator flaps or fibula flaps often entail some morbidities and complications. Sakai et al. first proposed a surgical alternative in 1991, namely a cortico-periosteal flap harvested from the medial condyle of the femur [1]. The medial femoral condylar flap applications are less invasive and complex than earlier methods, but are mainly used to treat ischemic fusion disorders of the carpal bone [1-6]. While evidently useful in select cases, the practice has limited applications since it cannot cover relatively large bone defects. Furthermore, its donor site is deep at the thick lower medial thigh, leading to a decreased surgical access.
In 1992, senior author (Isao Koshima) proposed the tibial artery perforator flap [7] and demonstrated via live surgery at the 2nd International Course on Perforator Flaps in Munich in 1999. We believe that this flap should now become an important method in the reconstructive armamentarium of lower limb defects. It has since been popularized for lower limb reconstruction. Based on our cadaveric studies, detailed analysis of the posterior tibial artery perforators revealed a peripheral vasculature which irrigates a variety of deep to superficial tissues including bone, periosteum, overlying adipose tissue and skin. This has allowed its clinical application as a periosteal, adiposal, and periosteo-cutaneous flap [7,8]. Building on this further, this article demonstrates the versatility of this perforator system in raising either a pedicled or free flap. Moreover, the anatomical feasibility of separating the TOP flap into its chimeric sub-units has allowed us to invent and report a new type of osseo-periosteal flap which is easier to elevate, covers larger areas and thereby has greater applications.
The advantages of the TOP flap in clinical practice are as follows: (1) a single perforator can sustain a relatively large periosteal flap; (2) reduced donor site morbidity compared to other flaps; (3) minimal anatomical perforator variation; (4) usefulness as a pedicled periosteal flap for tibial defects or non-unions; (5) the ability to use the TOP flap for free tissue transfer; (6) allowing access to cancellous tibial bone graft via the same surgical access; and (7) the ability to include a cutaneous chimeric component within the larger TOP flap.
Nevertheless, there remain some pitfalls. For instance, as the TOP flap is harvested at the distal perforator vasculature level, the vascular pedicle is small (less than 0.8 mm) and short. This makes it a difficult flap for inexperienced surgeons. Further, we generally avoid using the TOP flap on a child due to the risk of hindering the growth of the tibia following the stripping of its periosteal blood supply. Finally, careful consideration must be given when performing the TOP flap on females due to unaesthetic scarring.
In the long term, we believe that many of the current popular free vascularized bone flaps will be replaced by less invasive flaps leading to the best surgical outcomes with the least risks involved. Based on our clinical experience, the indications for the TOP flap include: (1) poorly vascularized tissue beds, either exposing or close to the bone, (2) augmenting vascular perfusion to a non-vascularized cancellous bone graft, (3) bony non-unions compounded by significant scarring, and (4) complex three-dimensional defects that require reconstruction of the skeletal framework, the overlying skin cover, and the intervening soft tissue. Each of these indications were discussed in case 1 to 4. Regarding osseous reconstruction, the TOP flap offers ideal bone substrate for the thin membranous bone for irradiated orbital walls, orbital blow-out/blow-in fractures, maxillary/alveolar/palatal clefts, malar hypoplasia, hemifacial hypoplasia, or Romberg’s disease. The chimeric TOP flap concept ensures three-dimensional freedom when dealing with complex or composite defects with multiple skin paddles, depending on the peripheral perforator tree [10].
The TOP flap can be used for various mild to moderately sized osteo-cutaneous defects with low morbidity and high technicality.
Received date: October 08, 2018
Accepted date: February 08, 2020
Published date: December 03, 2021
This study was presented in part at the Japanese Society of Head and Neck Cancers in Nagoya, Japan, in June of 1994; the First Annual Meeting of the International Course on Perforator Flaps in Gent, Belgium, in June of 1997; and the 41st Annual Meeting of the Japanese Society of Plastic and Reconstructive Surgery in Kyoto, Japan, in April of 1998.
The study is in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
© 2021 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
The authors present a revised application of the composite iliac crest bone free flap for hemimaxillectomy defects. This flap solely can give a support to the eye globe and the dental implants without osteotomies and titanium meshes. The upper ridge of the flap can be shaped to replicate the curvature of the orbital floor. The inner oblique muscle and the deep fascia can serve as an additional material for separation of the oral cavity from the nasal airway. Utilization of the Customized Contour Implants gives a surgeon a refining instrument for aesthetic correction of facial projection.
The likelihood of donor site ischemia following the harvesting of a fibula flap is extremely low, but it is potentially lethal if it occurs. The authors describe a case of ischemia of the lower extremity following a free fibula harvest for head and neck reconstruction. The authors discuss preoperative, intraoperative, and postoperative strategies to assist in diagnosing and managing risks associated with free fibula flap harvesting in this paper.
This case highlights the use of a bipedicled deep inferior epigastric perforator (DIEP) flap for reconstructing a massive 45 × 17 cm chest wall defect following bilateral mastectomy. By preserving abdominal musculature and utilizing preoperative computed tomographic angiography (CTA) for perforator mapping, the technique enabled tension-free bilateral microvascular anastomosis to the internal mammary arteries. The incorporation of submuscular mesh and minimal donor-site undermining maintained abdominal wall integrity. At six-month follow-up, no hernia or functional deficits were observed, and the patient reported high satisfaction on the BREAST-Q. This muscle-sparing strategy offers a viable alternative for large, midline-crossing chest wall defects where conventional flaps may be insufficient.
Koshima I, Imai H, Eldahshoury T, Yoshida S, Nagamatsu S, Yokota K, Harima M, Mizuta H, Yamashita S, Kannan R. Development of tibial osseo-periosteal flap for complex nonunions and bone defects. Int Microsurg J 2021;5(2):1. https://doi.org/10.24983/scitemed.imj.2021.00149