Objectives: Our group previously reported that the patients with type 1 diabetes had significantly lower pancreatic size than the non-diabetic controls (non-DCs). In the present study, we investigated the relationship between pancreas size and titers of islet autoantibodies in acute-onset type 1 diabetes and Oslowly progressive insulin-dependent diabetes mellitus (SPIDDM).
Methods: The pancreatic volume (PV) was measured by computed tomography in 71 patients with type 1 diabetes (32 acute-onset type 1 diabetes and 39 SPIDDM) and 39 age- and body mass index-matched non-DCs. Autoantibody titers against glutamic acid decarboxylase antibody (GADAb), insulinoma-associated antigen-2 autoantibodies (IA-2Ab), and zinc transporter 8 autoantibody (ZnT8Ab) were measured. The ratio of 100 × random C-peptide (ng/mL) to plasma glucose levels (mg/dL). C-peptide index (CPI) was measured as the residual β-cell function.
Results: The PV was significantly correlated with body weight in both the type 1 diabetes patients and the non-DCs. The PV index (PVI, PV/body weight) was decreased by 40% in the type 1 diabetes patients compared with that in the non-DCs. PVI in SPIDDM was reduced as the duration of diabetes progressed. No statistically significant correlation was found between the PVI and titers of GADAb and ZnT8Ab in acute-onset type 1 diabetes and SPIDDM. Among the patients with type 1 diabetes, PVI was significantly lower in patients with type 1 diabetes with a high titer of IA-2Ab (≥10 U/ml) compared with those with type 1 diabetes negative for IA-2Ab. The high IA-2Ab group had a high prevalence of acute-onset cases and titers of high GADAb and ZnT8Ab.
Conclusion: High titers of IA-2Ab reflect reduced pancreatic size in the type 1 diabetes patients, especially those with an acute-onset form of the disease. The potential mechanisms underlying the reduced pancreatic size might differ between acute-onset type 1 diabetes and SPIDDM.
Type 1 diabetes (T1D) is caused by autoimmune-mediated selective destruction of insulin-producing β-cells [1]. Exocrine abnormalities, however, have been reported in the morphology, pathology, and function of the T1D pancreas over the past decades [2-4]. Exocrine pancreatic inflammation and reduced pancreatic weight have been identified in patients with acute-onset T1D [5] and slowly progressive insulin-dependent diabetes mellitus (SPIDDM) [6]. Until now, the absence of insulinotropic effects on pancreatic exocrine tissue has been proposed as a possible explanation for the reduced pancreas size in T1D [2-4,7]. A previous report, however, has shown pancreatic exocrine atrophy in T1D patients with surviving β-cells [8].
Recent radiographic imaging modalities, including computed tomography (CT) [9,10] and magnetic resonance imaging [11-13], have also shown reduced pancreatic volume (PV) in patients with acute-onset T1D irrespective of the diabetes duration. Reduced pancreas size has recently been observed in both adults with newly diagnosed acute-onset T1D [12] and islet autoantibody-positive non-diabetic donors [14]. This observation suggests that the atrophy of the pancreas is likely to begin before the onset of T1D. Very recently, we also reported that the pancreatic volume assessed by CT was significantly reduced in the newly diagnosed acute-onset T1D patients, but not in the virus-induced fulminant T1D patients, when compared to non-diabetic controls (non-DCs) [15]. These findings suggest that the autoimmune process contributes to the pathogenesis of reduced PV in acute-onset T1D.
Although unlikely to be intrinsically diabetogenic, the circulating islet autoantibodies against glutamic acid decarboxylase antibody (GADAb), insulinoma-associated antigen-2 autoantibodies (IA-2Ab), zinc transporter 8 autoantibody (ZnT8Ab), and insulin (all of which are generated from destroyed β-cells) are strongly associated with the development of T1D. Among the several islet autoantibodies, GADAb has been measured worldwide by radioimmunoassay (RIA). Glutamic acid decarboxylase autoantibodies-radioimmunoassay (GADAb-RIA) is frequently used to diagnose autoimmune diabetes. Although the pathogenesis of T1D and residual β-cell function demonstrably differ between acute-onset T1D and SPIDDM, the reduced pancreatic size and the presence of circulating autoantibodies to islet autoantigens seem to be common to both forms of diabetes. There are limited data reported on the relationship between PV and the titers of GADAb, IA-2Ab, and ZnT8Ab in acute-onset T1D and SPIDDM.
The aim of the present study was therefore to investigate the relationship between islet autoimmunity and pancreatic size in the Japanese patients with T1D.
Participants
A total of 71 patients with T1D and 39 age- and body mass index (BMI)-matched non-DCs from the Showa University Hospital (Tokyo, Japan) were selected for enrolment in the present retrospective study. The study was conducted by adding 37 patients with T1D to a population we studied in our previous research [15]. Based on the diagnostic criteria and clinical information at diabetes onset, the patients with T1D were classified into two subtypes: acute-onset T1D (n = 32) [16] and SPIDDM (n = 39) [17].
The non-DCs with any one of the following criteria showed no clearly diagnosable evidence of diabetes: (1) blood glucose levels <110 mg/dL in fasting or <140 mg/dL after eating; and (2) HbA1c value of <6.0%. The exclusion criteria were as follows: (1) aged <20 years or >80 years; (2) any pancreatic disease (including acute pancreatitis); and (3) a history of alcohol abuse. The non-DCs in the current study were selected randomly from the patients who had undergone abdominal CTs for the following medical indications: screening for pulmonary embolism (n = 8), esophageal cancer (n = 7), gastric cancer (n = 4), lung cancer (n = 4), primary aldosteronism (n = 3), rectal cancer (n = 2), colon cancer (n = 2), appendicitis (n = 3), kidney stone (n = 1), inguinal hernia (n = 1), germ cell tumor (n = 1), liver hemangioma (n = 1), small intestine ileus (n = 1), nephrotic syndrome (n = 1), assessment and screening for acute abdominal pain (n = 1). The study protocol was approved by the Ethics Committee of the Showa University School of Medicine.
Measurement of Pancreatic Volume
CT images were acquired using a standard clinical protocol for abdominal/pelvis CT by a multidetector CT (LightSpeed Plus [4DAS] or LightSpeed Plus, GE Healthcare Japan, Tokyo, Japan; SOMATOM Definition AS+ or SOMATOM Sensation64, Siemens Healthcare, Erlangen, Germany). The CT scans were carried out at a tube voltage of 120 kVp, tube current of 180-200 mA (with partial auto-exposure control), tube rotation of 0.5-0.8 s/rotation, pitch factor of 0.6-1.5, and scanning time of 6.2-16 s. PV was calculated from contrast-enhanced or non-contrast-enhanced 5-mm axial CT images. Iodine contrast medium (Iomeron 300, Eisai, Tokyo, Japan; Omnipaque 300, Daiichi-Sankyo, Tokyo, Japan; Iopamiron 300, Bayer Health Care, Leverkusen, Germany) was intravenously injected at a rate of 3.0 mL/s by a power injector. The pancreas was manually outlined on axial image slices for semi-automatic calculation of the pancreatic volume using Ziostation2 (Ziosoft Inc., Tokyo, Japan) software, according to the methods previously reported [15]. The areas of the slices were summed throughout the volume of the pancreas. The images were initially analyzed by one diabetologist (A. Fukase) well trained in the measurement of PV and then confirmed by one experienced radiologist (H. Sasamori). The mean intraobserver coefficient of variation (CV) of the PV was 5.28 % (range, 0.39-9.26 %). The mean interobserver CV of the PV (five cases studied) was 3.31% (range, 1.24-5.09 %). The difference in the measurement of PV by both contrast-enhanced and non-contrast enhanced CT image (five cases studied) was 3.14% (range, 0.25-5.20 %).
Laboratory Analysis
GADAb titers were measured by RIA [18] and enzyme-linked immunosorbent assay (ELISA) methods [19], with cut-off values of 1.5 and 5.0 U/mL, respectively. IA-2Ab titer was measured by RIA, with a cut-off value of 0.4 U/mL (range, 0.4–50 U/mL) [20]. ZnT8Ab titer was measured by ELISA [21], with a cut-off value of 15 U/mL (range, 15–2000 U/mL). If the IA-2Ab and ZnT8Ab titers fell below the lower limits of detection, a fill value of 0.01 was used. The GADAb titer assessed by ELISA was the actually measured value, even if less than 5.0 U/mL. The glycated haemoglobin (HbA1c) content (%) was estimated as a National Glycohaemoglobin Standardization Program-equivalent value (%), as calculated by the following formula: HbA1c (%) = HbA1c (Japan Diabetes Society) (%) + 0.4% [22].
Assessment of the Insulin Secretion Ability
Serum C-peptide and plasma glucose levels were measured with immunoenzymometric assay and glucose oxidase methods, respectively. The ratio of 100 × random C-peptide (ng/mL) to plasma glucose levels (mg/dL). C-peptide index (CPI) was measured as the residual β-cell function.
Measurement of Islet-Associated Autoantibodies
Among a total of 71 patients with T1D, titers of GADAb-RIA and -ELISA were measured in 22 and 29 patients with acute-onset T1D and 28 and 38 patients with SPIDDM, respectively. Among the patients with acute-onset T1D, the measurements were performed by GADAb-RIA in 3, by GADAb-ELISA in 10, and by both methods in 19 patients. Among the SPIDDM patients, the measurements were performed by GADAb-RIA in 1, by GADAb-ELSA in 11, and by both methods in 27 patients.
IA-2Ab titer was measured in 30 patients with acute-onset T1D and 35 patients with SPIDDM. IA-2Ab and ZnT8Ab titers were measured in 25 patients (acute-onset T1D, n = 14; SPIDDM, n = 11) using samples that had been stored at -80°C until the analysis. These samples were taken when GADAb-RIA and -ELISA were measured simultaneously in December 2015.
Statistical Analysis
Comparisons between the groups were performed using the student’s t-test and the Mann-Whitney U test. Non-parametric correlations were identified using the Spearman’s rank correlation coefficient. The differences were considered significant at a two-tailed probability (P) value of <0.05. All the statistical analyses were conducted with JMP Pro 14.0 software (SAS Institute Japan Inc., Tokyo, Japan).
Characteristics of the Participants
Table 1 shows the patient backgrounds of the T1D group and the non-DCs group. There was no difference in the age or BMI between the T1D group and the non-DCs group. The patient age and the age at disease onset were significantly lower in the patients with acute-onset T1D than in the patients with SPIDDM. Body weight and BMI were significantly higher in SPIDDM than in acute-onset T1D. While no significant difference was found in the HbA1c value between acute-onset T1D and SPIDDM, the average (standard deviation: SD) HbA1c of T1D was 9.6 (2.2) %, indicating that a large proportion of the subjects had poor metabolic control. As expected, CPI was significantly lower in acute-onset T1D than in SPIDDM. GADAb-RIA titer tended to be higher in SPIDDM than in acute-onset T1D. GADAb-ELISA and IA-2Ab titers, in contrast, tended to be higher in acute-onset T1D than SPIDDM, but not to a degree reaching statistical significance. ZnT8Ab titers were significantly higher in patients with acute-onset T1D than in patients with SPIDDM.
/V2(1)1/Figure%201.JPG)
Figure 1. Relationship between the pancreatic volume and body weight. Pancreatic volume has been significantly correlated with body weight in patients with type 1 diabetes (panel A) as well as non-diabetic controls (panel B).
Analysis of Pancreatic Volume
As shown in Figure 1, the PV was significantly correlated with body weight in both the non-DCs and the patients with T1D. Thus, the pancreatic volume index (PVI, PV/body weight) was used for comparisons across multiple groups in the present study. The mean PVI in the patients with T1D was approximately 40% lower than that in the non-DCs (0.65 cm³/kg vs. 1.09 cm³/kg, P <0.0001, Figure 2A). The mean PVI was significantly lower in both the acute-onset T1D (0.57 cm³/kg) and SPIDDM (0.71 cm³/kg) compared to the non-DCs (Figure 2B).
A significant correlation was found between the PVI and the duration of diabetes in T1D and SPIDDM, but not in acute-onset T1D (Figure 3). All of the patients with acute-onset T1D were under intensive-insulin therapy alone. In contrast, the treatment of SPIDDM varied as insulin in combination with oral hypoglycemic agents (OHAs), insulin monotherapy, OHAs, and diet and exercise alone. Thus, we performed additional analysis to examine the correlation between PVI and the treatment for diabetes among the patients with SPIDDM. The PVI was significantly lower in insulin in combination with OHAs, insulin monotherapy, and OHAs compared to non-DCs. There was no difference in PVI between the diet-and-exercise-alone group and the non-DCs (Table 2).
/V2(1)1/Figure%202.JPG)
Figure 2. Pancreatic volume index of type 1 diabetes and non-diabetic controls. (A) The mean pancreatic volume index (PVI, pancreatic volume / body weight) is significantly lower in T1D (0.65 cm³/kg) than in non-diabetic controls (1.09 cm³/kg, P <0.0001). (B) The mean PVI is significantly lower in acute-onset T1D (n = 32, 0.57 cm³/kg) and SPIDDM (0.71 cm³/kg) than in non-diabetic controls (P <0.0001). The mean PVI is significantly lower in acute-onset T1D than in SPIDDM (P = 0.0218). The horizontal line represents the group mean value. SPIDDM, slowly progressive insulin-dependent diabetes mellitus; T1D, Type 1 diabetes.
/V2(1)1/Figure%203.JPG)
Figure 3. A significant inverse correlation is found between pancreatic volume index (pancreatic volume / body weight) and the disease duration in type 1 diabetes (panel A, r = -0.23, P = 0.049) and SPIDDM (panel C, r = -0.36, P = 0.026), but not in acute-onset type 1 diabetes (panel B). SPIDDM, slowly progressive insulin-dependent diabetes mellitus.
/V2(1)1/Figure%204.JPG)
Figure 4. Relationship between the pancreatic volume index and titer of GADAb by the RIA and ELISA method. No statistically significant correlation has been found between the pancreatic volume index (pancreatic volume / body weight) and titers of GADAb-RIA and -ELISA in acute-onset type 1 diabetes or SPIDDM. ELISA, enzyme-linked immunosorbent assay; GADAb, glutamic acid decarboxylase antibody; RIA, radioimmunoassay; SPIDDM, slowly progressive insulin-dependent diabetes mellitus.
/V2(1)1/Figure%205.JPG)
Figure 5. Relationship between the pancreatic volume index (pancreatic volume / body weight; PVI) and titer of IA-2Ab. A significant inverse correlation has been found between the PVI and titer of IA-2Ab in T1D patients (A). However, no statistically significant correlation has been found between PVI and IA-2Ab titer in acute-onset T1D patients (B) or SPIDDM patients (C). IA-2Ab, insulinoma-associated antigen-2 autoantibodies; N.S., not significant; SPIDDM, slowly progressive insulin-dependent diabetes mellitus; TID, type 1 diabetes.
Pancreatic Volume Index and Titers of Islet-Autoantibodies
No statistically significant correlation was found between the PVI and titers of GADAb-RIA and -ELISA in acute-onset T1D or SPIDDM (Figure 4). While a significant inverse correlation was found between the PVI and IA-2Ab titer in T1D (r = -0.259, P = 0.0372) in agreement with our previous research [15], no statistically significant correlation was found between the PVI and IA-2Ab titer in either acute-onset T1D or SPIDDM (Figure 5). In addition, no statistically significant correlation was found between the PVI and ZnT8Ab titer in total T1D, acute-onset T1D, or SPIDDM (data not shown).
Next, to further clarify the characteristics of the patients with higher titers of IA-2Ab, the patients with T1D were divided into three groups for PVI analysis by a nonparametric multiple comparison among them: a high-IA-2Ab-titer group (n = 12, IA-2Ab ≥10 U/ml), low-IA-2Ab-titer group (n = 22, 10 > IA-2Ab ≥0.4 U/ml), and IA-2Ab-negative group (n = 31, IA-2Ab <0.4 U/ml).
The PVI was significantly lower in the high-IA-2Ab group than in the IA-2Ab-negative group (0.50 cm³/kg vs. 0.70 cm³/kg, P = 0.0283) (Figure 6). The high-IA-2Ab group had a higher prevalence of acute-onset cases, higher titers of GADAb-ELISA and ZnT8Ab, and lower BMI when compared to the IA-2Ab-negative group (Table 3).
/V2(1)1/Figure%206.JPG)
Figure 6. Relationship between the pancreatic volume index and titer of IA-2Ab. The mean pancreatic volume index (pancreatic volume / body weight) is significantly lower in the high-IA-2Ab group (n = 12, IA-2Ab ≥10 U/ml) than in IA-2Ab-negative group (n = 31, IA-2Ab <0.4 U/ml). The horizontal line represents the group mean value. IA-2Ab, insulinoma-associated antigen-2 autoantibodies; N.S., not significant.
/V2(1)1/Figure%207.jpg)
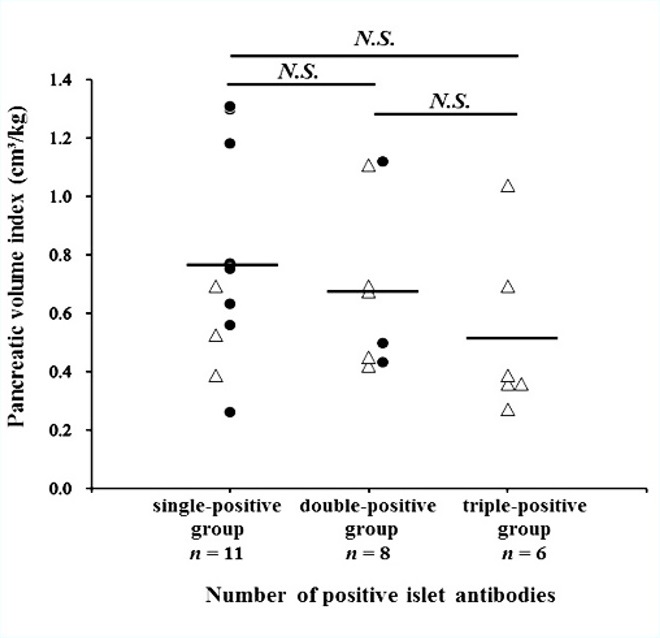
Figure 7. Relationship between the pancreatic volume index and stratification of the number of positive islet-autoantibodies. The mean pancreatic volume index (pancreatic volume / body weight) is not associated with the triple-positive islet autoantibodies (GADAb & IA-2Ab & ZnT8Ab) in patients with T1D (open triangles, acute-onset T1D; closed circles, SPIDDM). The horizontal line represents the group mean value. GADAb, glutamic acid decarboxylase antibody; IA-2Ab, insulinoma-associated antigen-2 autoantibodies; N.S., not significant; SPIDDM, slowly progressive insulin-dependent diabetes mellitus; T1D, type 1 diabetes; ZnT8Ab, zinc transporter 8 autoantibody.
Pancreatic Volume Index and Number of Positive Islet Auto-Antibodies
We investigated the correlation between the PVI and the number of positive islet autoantibodies (n = 25, GADAb [RIA and ELISA], IA-2Ab, and ZnT8Ab, Figure 7). The single-positive islet autoantibody group (GADAb-RIA, GADAb-ELISA, or both) included 3 patients with acute-onset T1D and 8 patients with SPIDDM. The double-positive islet autoantibodies group (GADAb & IA-2Ab, n = 7; GADAb & ZnT8Ab, n = 1) included 5 patients with acute-onset T1D and 3 patients with SPIDDM. All the patients in the triple-positive islet autoantibodies group (GADAb & IA-2Ab & ZnT8Ab) had acute-onset T1D. The mean PVI tended to decrease as the number of positive islet autoantibodies increased, but the differences in the PVI among the groups were not significant.
Comparison of the Pancreatic Volume Index Between the GADAb-ELISA (+) and GADAb-ELISA (-) SPIDDM Patients Who Tested Positive for GADAb by RIA
From December 2015, the method for measuring GADAb was switched from the RIA method to the ELISA method in Japan. Although the commercially available GADAb-ELISA is reported to have higher specificity for the diagnosis of T1D compared with the GADAb-RIA in western countries [23], approximately 30% of the SPIDDM patients who test positive on GADAb-RIA are reported to test negative on GADAb-ELISA [24].
To compare the PVI between the GADAb-ELISA (+) group and -ELISA (-) group, we analyzed the PVIs of 26 participants in whom GADAb-RIA and -ELISA were measured simultaneously (Table 4). Through this analysis, 13 (50%) of the 26 patients with SPIDDM were found to be negative in the GADAb-ELISA measurement (1 patient who tested negative in both the RIA and ELISA measurements was excluded in this analysis). The GADAb-ELISA (-) group had a higher BMI and higher CPI when compared to the -ELISA (+) group. The GADAb-RIA titers were significantly lower in the GADAb-ELISA (-) group than in the -ELISA (+) group (average ± standard deviation, 4.36 ± 4.05 IU/mL vs. 2083 ± 6376 IU/mL, P = 0.0044).
The PVI was significantly lower in the GADAb-ELISA (+) group and -ELISA (-) group compared to the non-DCs group (Figure 8). No significant difference was found in the mean PVI between the GADAb-ELISA (+) group and -ELISA (-) group (0.67 cm³/kg vs. 0.79 cm³/kg, respectively, P = 0.3299).
/V2(1)1/Figure%208.JPG)
Figure 8. Comparison of the pancreatic volume index between GADAb-ELISA-positive and GADAb-ELISA-negative SPIDDM patients. The mean pancreatic volume index (pancreatic volume / body weight) is significantly lower in the GADAb-ELISA-positive group and -ELISA-negative group compared to non-diabetic controls (P <0.0001, P = 0.007, respectively). The horizontal line represents the group mean value. ELISA, enzyme-linked immunosorbent assay; GADAb, glutamic acid decarboxylase antibody; N.S., not significant; SPIDDM, slowly progressive insulin-dependent diabetes mellitus.
We recently reported an inverse correlation between IA-2Ab titers and PVI among the patients with T1D [15]. The association of PVI with islet autoantibody status (type, number, and methods) remains to be elucidated, however, in acute-onset T1D and SPIDDM. The present study has demonstrated two never-before-reported findings on the PVI of patients with acute-onset T1D and SPIDDM. First, the PVI was significantly reduced in T1D patients with high IA-2Ab titer (≥10.0U/ml) compared to the T1D patients who tested negative for IA-2Ab titer. These patients had more prevalence of acute-onset cases, GADAb-ELISA and ZnT8Ab in high levels, and less BMI, indicating phenotypic characteristics in acute-onset T1D. In addition, we found that neither GADAb titer, ZnT8Ab titer nor the number of positive islet autoantibodies were all associated with PVI in both the acute-onset T1D and SPIDDM patients.
Accumulating evidence suggests that the noninvasive imaging of the pancreas often reveals a reduced pancreas size in acute-onset T1D [5] and SPIDDM [6]. The clinical implications of this reduction in the size of the pancreas have yet to be fully understood. The earlier research on pathology has assumed that the mechanisms underlying the reduced pancreas size in T1D largely stems from a loss of insulinotropic effects acting on the exocrine pancreas, as insulin is a potent growth factor for the exocrine pancreas [2-4]. Several groups, however, have reported an absence of any relationship between the PV and residual β-cell function in the acute-onset T1D and SPIDDM patients [12,15]. The data from these reports suggest that the reduced PV of T1D (irrespective of subtypes) is unrelated to the loss of β-cell function.
Reduced pancreatic size has recently been observed in adults with newly diagnosed acute-onset T1D [12] and islet autoantibody-positive non-diabetic donors [14]. In addition, Wiberg et al. reported that the histopathological finding of CD45+ cells infiltration were shown in exocrine pancreas and those cells might contribute to the development of exocrine autoantibodies in the islet-autoantibody positive non-diabetic donors [26]. These suggest that the exocrine pancreatic atrophy and exocrine inflammation might stem from autoimmunity development against both pancreatic endocrine and exocrine cells and precede the onset of acute-onset T1D. Among the different islet autoantibodies, IA-2Ab is well recognized to be a more specific marker of autoimmune-mediated destruction of beta cells than GADAb [20] or ZnT8Ab [21]. Considering that higher titers of IA-2Ab are associated with reduced PVI in T1D, especially acute-onset T1D with high titers of GADAb and ZnT8Ab, we presume that the autoimmune pathogenesis of the disease contributes to reduced PVI in the patients with acute-onset T1D.
Second, the PVI reduction is found to take place over time in SPIDDM, although it begins at or near the disease onset and is minimally altered in the patients with acute-onset T1D who have suffered the disease for as long as several decades. Our group previously reported that SPIDDM patients had reduced pancreatic weight, along with intraepithelial neoplasia (PanIN) lesions accompanied by inflammatory changes [6]. The acinar cells around the PanIN in these subjects were extensively injured or completely lost, with extensive infiltration of inflammatory cells into the area, and ultimately lobular atrophy (unpublished observations by T. Fukui). According to descriptions of postmortem pancreatography by Suda et al., the findings of PanIN increase in frequency as the age of the individual advances [27]. Given that SPIDDM is characterized by a late age of onset and slowly but progressive functional impairment of β-cells [17], a ductcentric lobular atrophy that progressed with time over the course of the disease independently of islet auto-immunity and residual β-cell function might have partly contributed to the reduced PVI of the SPIDDM patients observed in the current study.
Another novel finding from the current study was a similar reduction of the PVI in SPIDDM patients with GADAb-RIA (+) / GADAb-ELISA (+) and those with GADAb-RIA (+) / GADAb-ELISA (-), when compared to the PVI of the non-DCs patients. Thirteen (50%) of the 26 GADAb-RIA-positive SPIDDM patients in the present study turned out to be GADAb-ELISA (-), which agreed with the previous reports [24]. While the reason for this seroconversion in SPIDDM patients has yet to be explained, the CPI of the patients who tested negative on GADAb-ELISA was significantly preserved compared to the CPI of the patients who tested positive (1.21 ± 0.66 vs. 0.50 ± 0.35, P = 0.0066). The disease of the patients who tested negative on GADAb-ELISA, it would seem, ran a less progressive course than the disease of the patients who tested positive.
There are several limitations in the current study. First, we examined a relatively small sample, and the retrospective and cross-sectional design of the study limited the precision of the results. Second, our outpatient results should be interpreted with caution, as the CT scans and measurements of islet-autoantibodies were not carried at the same time. Third, among islet-autoantibodies, we were not able to clarify the reason why titers of IA-2 Ab alone was associated with reduced PV in patients with T1D. However, since these pa-tients with high IA-2Ab titer had GADAb-ELISA and ZnT8Ab in high levels, titers of IA-2Ab was presumed to be indicative of reduced PV in autoimmune-related T1D. Finally, the study shed no light on the precise mechanism(s) underlying the PVI reduction in acute-onset T1D or any explanation as to why the reduction took place irrespective of the disease duration. A large cohort of patients at high risk of developing acute-onset T1D would be required to clarify the mechanism(s) of the decreased PVI in acute-onset T1D.
A higher titer of IA-2Ab reflects a reduced pancreatic size in the patients with T1D, especially in those with the acute-onset form of the disease. The potential mechanisms underlying the reduced pancreatic size might differ between acute-onset T1D and SPIDDM.
Received date: December 03, 2018
Accepted date: December 31, 2018
Published date: February 25, 2019
This report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no financial or other conflict of interest relevant to this article, which is the intellectual property of the authors.
We greatly appreciate Professor Tetsuro Kobayashi of the Division of Immunology and Molecular Medicine, Okinaka Memorial Institute for Medical Research for the excellent advice for the current study.
Results from this study were presented at the 61st annual meeting of Japan Diabetes Society in Tokyo on May 24-26, 2018.
© 2019 The Author (s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
The authors examined the appropriateness of an IIP, which was modified to fit the Japanese population, who were usually less obese and less insulin-resistant. This IIP maintained blood glucose levels within the target range in patients who underwent open-heart surgery as equally well as the previous empirical therapy. These findings confirm the efficiency and safety of our IIP with less burden in blood glucose management of Japanese patients.
This article presents a crucial case report on potential wound healing complications linked to fremanezumab, a calcitonin gene-related peptide-targeting antibody for migraine prevention. It documents the first known instance of delayed wound healing following a free flap breast reconstruction, underscoring the need for heightened clinical vigilance and individualized patient assessment in perioperative settings. Highlighting significant safety data gaps, the report advocates for comprehensive research and rigorous post-marketing surveillance. The findings emphasize the importance of balancing the risks of delayed wound healing with the need for effective disease control, especially when using biologic agents for chronic conditions. This article is essential for medical professionals managing patients on biologic therapies, offering critical insights and advocating for a personalized approach to optimize patient outcomes. By presenting novel observations and calling for further investigation, it serves as a vital resource for enhancing patient care and safety standards in the context of biologic treatments and surgical interventions.
In this study, Fukase et al. examined the relationship between pancreas volume (PV) and titers islet autoantibodies in acute-onset T1D and SPIDDM. The PV was measured by CT in 71 patients with T1D (32 acute-onset and 39 SPIDDM) and 39 age- and BMI-matched non-diabetic controls. They found that patients with T1D with high titer of IA-2Ab showed reduced PV compared with those without. Changes in exocrine pancreas in patients with T1D is currently an active research area and of importance to explore the mechanisms of T1D development. Although the retrospective study design and small sample size were limitations of the study, the findings were clearly presented, and the manuscript was well-written. However, some issues need to be addressed further.
The authors showed that high titers of IA-2Ab reflect reduced pancreatic size in type 1 diabetes patients, although there was no significant correlation between the pancreatic volume index (PVI) and titers of GADAb and ZnT8Ab in acute-onset type 1 diabetes and slowly progressive insulin-dependent diabetes mellitus (SPIDDM). The findings are novel and important, but there are several issues of concerns.
Fukase A, Fukui T, Mori Y, Nagaike H, Goto S, Hayashi T, Yamamoto T, Ohara M, Sasamori H, Hirano T. Relationship between islet autoantibodies and pancreatic volume in type 1 diabetes in Japanese population. Diabetes Endocrinol 2019;2(1):1. https://doi.org/10.24983/scitemed.de.2019.00101