Radiation-induced rectal perforation during the treatment of locally advanced rectal cancer (LARC) is very rare. Symptomatic perforation is diagnosed clinically and requires urgent intervention. However, asymptomatic perforation may be found incidentally on imaging and this can be easily confused with residual/progressive disease. Magnetic resonance imaging (MRI) may help in correct diagnosis and choosing the right line of treatment. Case 1: A 32-year-old gentleman was operated in another centre for upper rectal adenocarcinoma (T3, N0) without adjuvant therapy. He was treated with chemoradiation therapy for anastomotic site recurrence after nine months. He was referred to our centre for residual presacral disease on computerized tomography. However, MRI of the pelvis showed the mass to be heterogeneous with air pockets on T2 sequence and a fistulous communication between rectal lumen and mass. This indicated complete resolution of recurrence with a perforation at the previous colorectal anastomotic site. Case 2: A 33-year-old lady with mid rectal adenocarcinoma was treated with short course of radiotherapy and systemic chemotherapy at our centre. On reassessment using MRI, local progression was suspected. But, on careful review of MRI, a tumour site perforation was found, which was communicating with mesorectal fat. She underwent surgery for primary disease and this finding was confirmed intra-operatively. In conclusion, differentiating rectal perforation from recurrence or progressive disease during radiation for LARC is difficult due to lack of specific imaging characteristics. However, the presence of breach in the rectal wall and fistulous communication between mass and rectal lumen on MRI are confirmatory findings of perforation and thus help in choosing the right treatment.
Radiation forms a vital part of neoadjuvant treatment in locally advanced rectal cancer (LARC) and recurrent rectal cancers. The adverse effects of radiation are well recognized; however, radiation-induced perforation at the tumour site is very rare and is poorly understood. A symptomatic rectal perforation requires an emergency surgical intervention. However, it may present silently and can give rise to suspicion of disease progression and/or residual disease on imaging. Here, we present two cases of silent perforations. Both gave rise to a considerable diagnostic dilemma, which was resolved by careful evaluation with magnetic resonance imaging (MRI).
Case 1
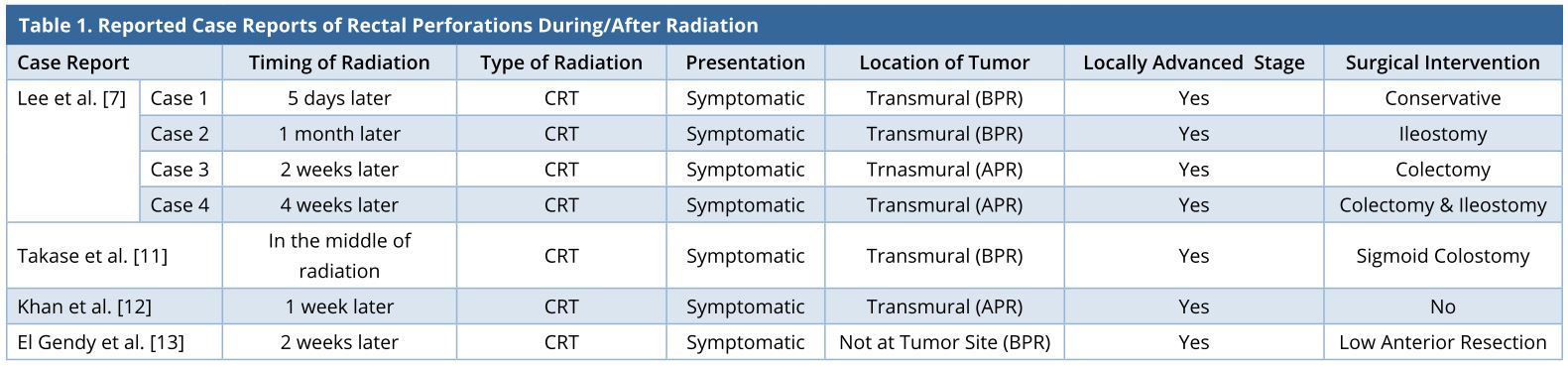
A 32-year-old gentleman was evaluated in another centre for bleeding per rectum. There were no symptoms of obstruction or distant spread and no significant family history. Clinical examination did not reveal anything significant. Colonoscopy revealed a rectal growth at 10-15 cm from anal verge. Biopsy was performed, and the result showed moderately differentiated adenocarcinoma. Computerized tomography (CT) scan of the abdomen and thorax showed a localized rectal growth without perirectal fat stranding or nodes and no distant metastasis. Serum carcinoembryonic antigen (CEA) was 8.3 ng/ml. He underwent upfront open anterior resection with covering ileostomy. Postoperative recovery was uneventful. Histopathology revealed moderately differentiated adenocarcinoma with extracellular mucin pools at places. Circumferential resection margins (CRM) and proximal and distal resection margins were free. Pathological staging was T3, N0 (out of 22 nodes). Adverse histological features were absent. He did not receive any adjuvant treatment. Nine months after the surgery, he developed pain abdomen. Clinical examination did not yield anything significant. Serum CEA was 7 ng/ml. CT scan showed a 7 x 4.8 x 5.6 cm lesion posterior to the rectum at the anastomotic site with preaortic nodes of 2.3 x 1.6 cm size, with no other metastases. He received local radiation (50 Gy) with capecitabine followed by 2 cycles of capecitabine and oxaliplatin (CAPOX). Post-treatment showed that he was clinically asymptomatic with a falling CEA trend (7 to 4.8 ng/ml). A repeat CT scan done 3 months later showed persistent 5 x 5 cm, well-defined, enhancing, hyperdense mass in the presacral region abutting the posterior wall of rectum (Figures 1 and 2). He was then referred to our cancer centre. No remarkable abnormalities were observed in the digital rectal examination.
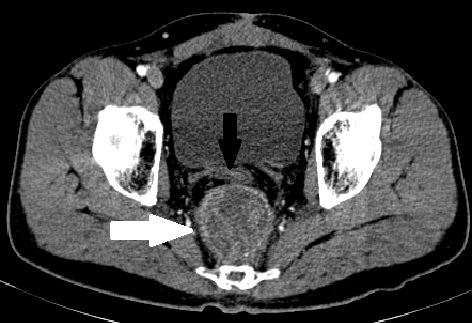
CT-guided biopsy of the mass was inconclusive. An MRI was done to rule out residual disease (Figures 2, 3) and it showed a heterogeneously T2-intense presacral mass communicating with the rectal lumen, suggestive of breakdown at the site of anastomosis (Figure 3). It was concluded that the recurrent tumour at the anastomotic site had responded completely and eventually led to a subclinical anastomotic leak. The patient was advised close follow-up. However, four months later, the patient developed a diffuse peritoneal disease, which was not amenable to cytoreductive surgery. He was started on palliative chemotherapy with FOLFIRI (folinic acid, fluorouracil, and irinotecan) regimen. At 6 months follow-up, the patient was alive with disease.
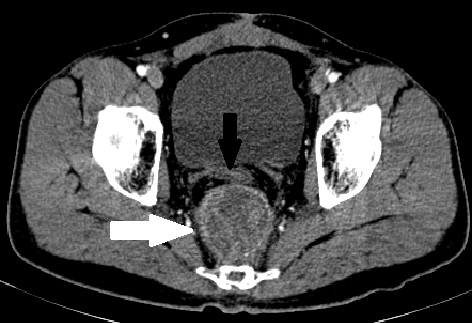
Figure 1. Contrast-enhanced CT scan image at the level of the bladder, done after CRT for local recurrence, showing a 5 x 5 cm well-defined heterogeneously enhancing (white arrow) mass posterior to the rectal tube (black arrow). CRT, chemoradiation; CT, computerized tomography
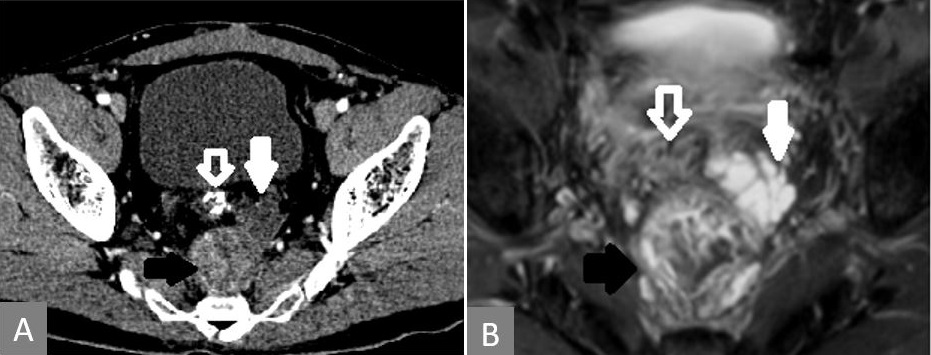
Figure 2. T2-MRI showing that the enhancing lesion seen on CT is heterogeneous on MRI (solid black arrow) and the nonenhancing lesion in CT is mucin deposit, which is T2 intense (solid white arrow). Rectal tube indicated with white line arrow in both images. (A) CT showing the enhancing mass in the presacral region (solid black arrow) along with another nonenhancing mass anterolateral to it (solid white arrow). (B) MRI of the lesion at the same level. CRT, chemoradiation; CT, computerized tomography; MRI, magnetic resonance imaging.
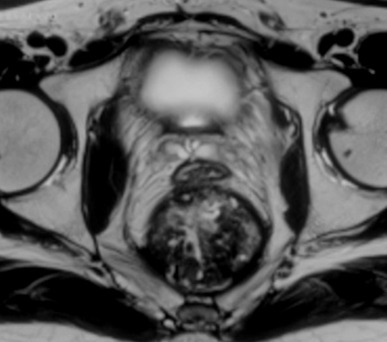
Figure 3. Breach in the rectal wall and communication between the presacral mass and the rectal tube.
Case 2
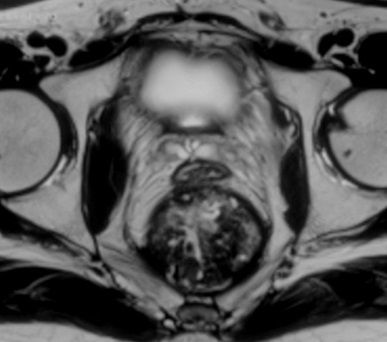
A 33-year-old premenopausal female presented with complaints of constipation and bleeding per rectum for 4 months. There were no symptoms of distant spread. There was no history of rectal or gynaecological cancers in the family. Digital examination showed growth at 5 cm from the anal verge. Endoscopic biopsy confirmed mucinous adenocarcinoma. CT scan of abdomen and thorax showed a suspicious para-aortic node and a suspicious liver metastasis. CEA was 11.4 ng/ml. MRI of pelvis showed a transmural tumour in the mid-rectum with the involvement of the CRM, but the vaginal wall appeared free. She underwent short-course radiation (25 Gy, 5 fractions, over 5 days) followed by 4 cycles of chemotherapy with 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). Post-radiation MRI done 5 weeks later showed a new 5.5 x 6.8 x 5.8 cm heterogeneous exophytic mass arising from the left posterolateral wall of the rectum, displacing it (Figure 4). On T2 sequence, there were bright areas inside the lesion, suggestive of mucin collection. Careful evaluation of MRI images revealed a breach in the rectal wall communicating rectal lumen with the mass. Heterogeneity of the mass was due to necrotic debris and air pockets secondary to perforation. The mesorectal fascia was involved in the anterior planes. The liver lesion turned out to be a simple cyst and the para-aortic node showed complete resolution.
The patient underwent anterior resection with hysterectomy for R0 resection along with diversion transverse colostomy. Intraoperatively, it was observed that there was a contained perforation at the tumour site posteriorly without peritoneal contamination. Postoperatively, the patient developed anastomotic leak necessitating a Hartmann’s procedure. Histopathology showed no residual tumour. However, 2 out of 16 nodes showed mucinous adenocarcinoma deposits with perinodal extension. There were pools of extracellular mucin in mesorectal fat. She received adjuvant chemotherapy of 6 cycles CAPOX. When she came back for stoma closure, she had suspicious deposits on the anterior peritoneum. On exploration, there was extensive peritoneal disease not amenable to surgical treatment. Hence, stoma reversal was not done. She was started on palliative chemotherapy. At 28 months follow-up, the patient is alive with disease.
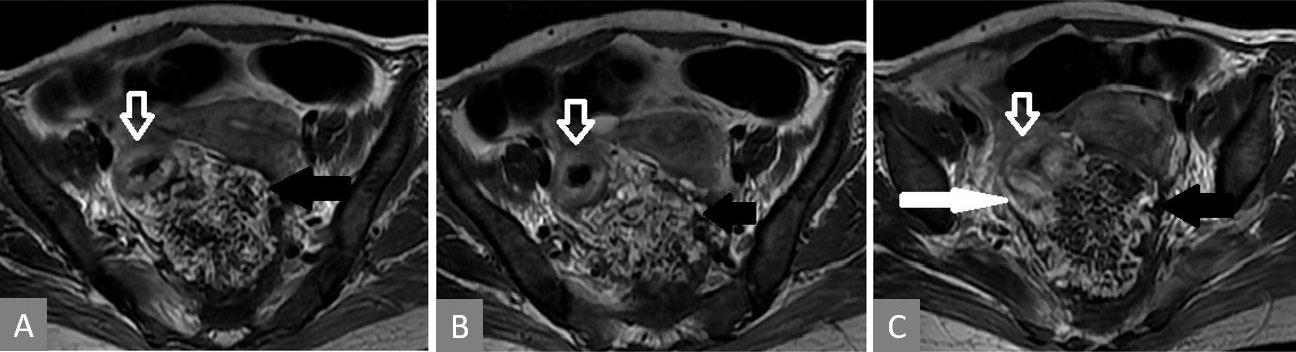
Figure 4. Serial T2 weighted MRI images (in cranio-caudal direction). Thickened rectal wall (white hollow arrow) with heterogeneous exophytic mass (solid black arrow) pushing it (A, B, and C). Posterior breach is observed in the rectal wall (white solid arrow in panel C).
Acute adverse effects of neoadjuvant radiation in LARC include gastrointestinal toxicity, genitourinary, neurological complications, and delayed wound healing. However, most of these are self-limiting [1]. Severe bleeding, strictures, perforation, fistula, and bowel obstruction occur in the chronic phase, which may not become apparent for months to years [2]. Rectal perforation and anastomotic breakdowns are very rare. Only a handful of case reports are seen in the medical literature (Table 1).
APR, above peritoneal reflection; BPR, below peritoneal reflection; CRT, chemoradiation.
All the reported cases of rectal cancer perforation have been T3 or T4 tumours with transmural involvement. It seems to be a combination of tumour characteristics and radiation effects that lead to perforation since most of the transmural tumours do not perforate. A larger tumour has a hypoxic and hypovascular centre leading to central necrosis [3,4], while radiation accelerates cell lysis in well-perfused peripheral zone, thus leading to weakening of the wall. However, there is anecdotal evidence that larger and undifferentiated tumours may be less responsive to radiation [5,6].
Mucinous variety of adenocarcinomas, as seen in both the cases presented here, are known to be less responsive to neoadjuvant radiation and have poor outcome overall. Mucin is produced and stored under pressure, which leads to mucin perforating rectal wall and migrating into the surrounding space wherever the wall weakens.
The time period of presentation has been varied (Table 1). All the reported cases were symptomatic, thus requiring some form of intervention. In Case 1, there was a diversion stoma, and in Case 2, the perforation might have been small, hence both the cases were clinically silent. Complication of rectal perforation could be dangerous. Out of four patients in the report of Lee et al., two died in the preoperative period [7].
Short course radiotherapy (SCRT) is said to be safer than long course radiotherapy [8,9], but rectal perforation after SCRT is also reported [10]. The potential risks for CRT-related rectal cancer perforation may include the presence of diverticula, collagenases, and tumour ulceration [11].
Small bowel obstruction, fistula, cystitis, and chronic diarrhoea are some of the late side effects of re-irradiation [12-18]. Fistulae in the lower pelvis are often associated with recurrent disease and may be exacerbated by tumour destruction [17]. Here, in Case 1, radiotherapy (RT) was used only after pelvic recurrence. The presacral recurrence showed excellent response to RT, but later images showed a leak from the rectum, most probably from the anastomotic line. This might be due to RT-induced ischemic changes of the normal rectal tissue.
In asymptomatic cases, CT and MRI are essential tools to differentiate recurrence from perforation and other benign findings, as fine-needle aspiration or biopsy are not always helpful. However, they still have limitations. The post-rectal surgery CT picture can be varied, wherein a presacral mass could be anything from normal postoperative changes to hematoma, abscess, uterus or enlarged prostate, and local recurrence. It is vital to remember that the normal postoperative changes that occur in the midline become well-defined over a period of time [19]. Similar findings were seen in Case 1. The size of the mass and its attenuation value could not be used to conclusively diagnose the recurrence [20,21].
MRI is more sensitive than CT in detecting recurrences but not without caveats. As seen in both the cases here, a recurrent tumour typically shows a T2 hyperintense signal but so do hematoma, granulation tissue, and inflammation [22]. A T2 hypointense lesion creates further confusion.
In both the cases, the diagnosis of perforation was possible due to the demonstration of communication between the mass and rectal lumen on MRI, the absence of mucin in the mass while it was present in the surrounding sites along with normal CEA, and lack of symptoms. Prophylactic diversion stoma for tumours above peritoneal reflection is suggested by some [12], but doing it for all cases for such a rare complication is not justified.
Perforation of rectal tumour is a rare event during the treatment of rectal cancer. It may present asymptomatically and after a considerable time lapse, incidentally found on imaging. No specific imaging characters are defined to differentiate rectal perforation from recurrence or progressive disease, however, identifying a breach in the rectal wall and fistulous communication between mass and rectal lumen on MRI are confirmatory findings of perforation and thus help in choosing the right treatment. There are two major learning points in the report: (1) rectal tumour perforation during radiation of rectal cancer is rare but can occur and create diagnostic dilemma, especially in clinically silent cases; (2) a well-timed MRI helps in differentiating perforation from a residual/progressive disease.
Received date: September 15, 2017
Accepted date: January 12, 2018
Published date: January 26, 2019
None
None
© 2019 The Author (s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
The high incidence of brain metastasis and the comparison of metastatic and non-metastatic phenotypes indicate an active crosstalk of brain metastatic breast cancers with the BBB. Certain miRNAs and serpins are regulatory molecules in defining the metastatic potential of breast cancers. Targeting these factors that favor the metastatic microenvironment may provide future therapeutic interventions for the brain metastasis of breast cancers.
This study reviews the reconstruction modalities within the multidisciplinary approach for chest wall reconstruction. The authors have proposed an algorithm that is formulated based on their experience to manage chest wall defects following cancer resection.
This is a case report with a comprehensively systematic review on juxtacortical chondrosarcoma in the head and neck area (HNJCS). According to the study, only nine cases of HNJCS have been adequately described. HNJCS have relatively consistent clinical and diagnostic profile regardless of location in the body. Surgical management yields excellent outcomes with low recurrence rates.
Total thyroidectomy and adjuvant RIT followed by a suppressive dose of levothyroxine are the established therapeutic procedures of choice for DTC. The treatment of DTC has changed from a one size fits all standard to a more individualized approach. The use of less complete surgery as well as decision to use RIT and the dose administered are to be considered carefully in the treatment of DTC. Surveillance for very low risk DTC is an acceptable option. The aim to lower morbidity, lower the cost of treatment and improve patient quality of life is attainable using these principles.
The study aims to assess the predictive values of certain psychological factors on the quality of life in patients with Head and Neck Cancer after radiotherapy. The authors conclude that the identification and the understanding of the depressive symptoms of patients, their beliefs about their illness as well as their coping strategies may provide the basis for timely implementation of appropriate intervention that may improve the quality of life in patients.
This article pioneers the first electrophysiological evidence of vocalis muscle reinnervation following recurrent laryngeal nerve (RLN) repair in humans, marking a significant advancement in nerve repair science. By utilizing intraoperative nerve monitoring, the study confirms successful reinnervation through clear electromyographic responses, establishing a critical benchmark in RLN repair validation. This research is crucial for medical professionals as it highlights the importance of precise surgical techniques and rigorous postoperative monitoring, promising enhanced recovery and improved vocal cord function. The findings offer a fresh perspective on nerve regeneration, providing renewed hope for patients suffering from vocal cord paralysis. This study is essential reading for its innovative approach and its potential to reshape surgical and diagnostic practices. It engages readers by blending scientific rigor with a compelling narrative of medical advancement and patient hope.
Controversies have recently arisen regarding post-operative haemorrhagic complications in relation to the surgical procedures adopted for tonsillectomy. The authors set out to verify the relationship between surgical techniques and post-operative haemorrhage based on the analysis of data derived from multi-centric studies.
This study examined two surgical techniques commonly used for turbinate reduction (i.e., submucous resection and partial excision) and their associated complications in functional nasal surgery patients. So far, there has been no direct comparison of these two methods with endpoints of epistaxis and nasal congestion. It was found neither technique was statistically significantly different from the other, so both are clinically useful with a low incidence of postoperative epistaxis.
This article presents a crucial case report on potential wound healing complications linked to fremanezumab, a calcitonin gene-related peptide-targeting antibody for migraine prevention. It documents the first known instance of delayed wound healing following a free flap breast reconstruction, underscoring the need for heightened clinical vigilance and individualized patient assessment in perioperative settings. Highlighting significant safety data gaps, the report advocates for comprehensive research and rigorous post-marketing surveillance. The findings emphasize the importance of balancing the risks of delayed wound healing with the need for effective disease control, especially when using biologic agents for chronic conditions. This article is essential for medical professionals managing patients on biologic therapies, offering critical insights and advocating for a personalized approach to optimize patient outcomes. By presenting novel observations and calling for further investigation, it serves as a vital resource for enhancing patient care and safety standards in the context of biologic treatments and surgical interventions.
This narrative review advances the management of radiation-induced dysphagia in head and neck cancer treatment by integrating advanced imaging, precision dosimetry, and structured rehabilitation. Beyond focusing solely on dysphagia-optimized intensity-modulated radiotherapy, it explores a variety of approaches: MRI and CT provide detailed anatomical insights, while proton therapy offers a less toxic alternative, and early rehabilitation preserves swallowing function. This holistic model prioritizes patient quality of life, detailing how techniques like Do-IMRT reduce radiation to critical structures like the pharyngeal constrictors and larynx, maintaining effectiveness. The review advocates a multidisciplinary approach, encouraging collaboration among oncologists, radiologists, and therapists to enhance long-term patient well-being and promoting further research to elevate care standards.
Vessel-compromised neck is a challenge for the microvascular surgeon planning for a free tissue transfer. This study describes the authors’ experience regarding a successful free tissue transfer in a vessel-compromised neck. The authors also propose an algorithm for management of vessel-compromised neck.
This narrative review advances the management of radiation-induced dysphagia in head and neck cancer treatment by integrating advanced imaging, precision dosimetry, and structured rehabilitation. Beyond focusing solely on dysphagia-optimized intensity-modulated radiotherapy, it explores a variety of approaches: MRI and CT provide detailed anatomical insights, while proton therapy offers a less toxic alternative, and early rehabilitation preserves swallowing function. This holistic model prioritizes patient quality of life, detailing how techniques like Do-IMRT reduce radiation to critical structures like the pharyngeal constrictors and larynx, maintaining effectiveness. The review advocates a multidisciplinary approach, encouraging collaboration among oncologists, radiologists, and therapists to enhance long-term patient well-being and promoting further research to elevate care standards.
Following corrections and additions are required to uplift the quality of the manuscript.
In case 2, how could have the risk for peritoneal disease, that developed later, be minimized? Please justify performing hysterectomy along with anterior resection in case 2. If it was upon patient's request, mention it.
ResponseRisk of peritoneal dissemination at a later stage can be minimized by achieving R0 resection and avoiding spillage. In case 2 hysterectomies was done along with anterior resection to ensure R0 resection and there was no spillage intra operatively. The role of prophylactic peritonectomy and HIPEC (hyperthermic intra peritoneal chemotherapy) in locally advanced rectal cancer is still under evaluation and cannot be used as a standard of care to reduce peritoneal recurrences. Advanced T stage , N stage , non radical surgery for primary tumor are known factor increasing the risk of metachronous peritoneal disease.Hysterectomy was done along with anterior resection to ensure R0 resection because post radiation MRI scans had shown involvement of mesorectal fascia anteriorly. ( this point has been added to the manuscript ,line 3 pages 6, and first line page 7).
Is there a role of surgical intervention in assymptomatic rectal perforation after being diagnosed? What is the prognosis of such patients?
ResponseWe believe that if the patient has not developed overt signs of perforation and there is no direct and extensive contamination of peritoneal cavity, surgical intervention can be safely avoided. However if there is spillage of tumor contents into the peritoneal cavity, then a diversion stoma/Hartmann’s and probably reassessment of treatment plan is necessary. In the presence of peritoneal disease, if it’s possible to complete cytoreduction combined with HIPEC , the overall survival can be as long as 20-30 months. Survival after incomplete cytoreduction and systemic chemotherapy is about 8-12 months.
The paper is interesting and contains useful clinical information about the diagnosis and treatment of the rectal fistulas in patients with rectal cancers treated previously with RT or CRT pre-adjuvant to surgery in two Cases described.
ResponseThank you for your comments. We want to stress up on the fact that asymptomatic rectal perforation can be easily confused with disease progression after radiation, in rectal cancer, if careful evaluation of imaging is not done.
The length of the paper is excessive and I would recommend shortening about 30 to 50% of the total.
ResponseWe have reduced the length of the manuscript and redundant parts, thereby improving the readability of the content. We hope this makes the message of the paper clear to the audience. Abstract was reduced from 283 words to 266.Main manuscript is reduced from 2138 words to 1833 (this includes all the tables and legends). This amounts to 20-25%% reduction is the size. We believe, reducing it any further would make it impossible for us to accommodate the suggestions given by other reviewers and might lead to loss of valuable information.
The Table including similar cases collected of the literature is very informing, but you must explain the meaning of the acronyms BR and AR.
ResponseAcronyms have been explained at the bottom of the table.
The Figures are the great quality and very informing about the superiority of MRI images compared to those of CT.
ResponseThe purpose of the paper is to stress on the need for good quality MRI of the pelvis in rectal cancers, in primary as well as recurrent settings, since it gives so many additional details about the disease biology and extent.
The discussion is informative, but redundant, and must be shortened. In the references of the papers from the Journals is not usual to include the date and the month of the publication, according to Vancouver Recommendations.
ResponseThe discussion has been shortened considerably. Necessary corrections have been made to the reference list.
You must review your English and try to improve it with and English native teacher.
ResponseWe have revised the manuscripts to the best our ability and we have also taken the help of an expert language editor in the process. We hope the language of the manuscript lives up to the expected standards.
The manuscript is well written, has important clinical message, and should be of great interest to the readers.
ResponseThank you very much for your encouraging words. We hope by the way of this paper more people will understand the unique phenomena of asymptomatic rectal perforation in rectal cancer treatment.
Authors say that there are previous reports about this subject. A record table about previous clinical cases plus these two could be added?
ResponseThough there are reports of rectal perforation during and after neo adjuvant radiation for rectal cancer, asymptomatic rectal perforations have not been reported earlier. A table of reported cases is already present in the manuscript.
Kammar P, Ankati SK, Engineer R, Patil P, Ostwal V, Saklani A. Asymptomatic rectal perforation after radiation for rectal cancer: A diagnostic dilemma and role of MRI. Ann Case Rep Images 2019;2(1):1. https://doi.org/10.24983/scitemed.acri.2019.00095