Cutaneous leishmaniasis and basal cell carcinoma (BCC) are two common clinical entities in Yemen. The hypothesis of concurrent occurrence of leishmaniasis and malignancy has been reported from several regions worldwide, and in many of them, leishmaniasis has been indicated as a risk factor for the development of malignant lesions with a direct or/and indirect involvement. Hereby, the author reports a case of BCC (Clark level IV) that had been developed on an active lesion of mucocutaneous leishmaniasis, supposing that carcinogenesis in the current case was triggered by Leishmania parasites and influenced by sun’s ultraviolet radiation exposure. Further studies with appropriate methods and experimental approaches are recommended to carefully address these challenging hypotheses.
Cutaneous leishmaniasis (CL), a chronic granulomatous inflammation caused by an infection of intracellular Leishmania parasite, is a worldwide challenging disease with significant endemicity in Yemen. Mucocutaneous leishmaniasis (MCL) is the most prevalent form of CL in Central Yemen, where the rural population is predominantly affected, particularly the children and the women. Leishmania major is referred to as the principal causative agent in these cases [1].
Basal cell carcinoma (BCC) is the most common histological type of skin cancer that prevails in Yemen, where malignant skin tumors account for 10% of all malignancies. Its prevalence is high among the elderly and the most common lesion sites are on the sun-exposed skin [2,3].
BCC rarely metastasizes; however, it could spread into nearby tissues and organs. Development of BCC is strongly associated with several risk factors, such as family history, intense ultraviolet exposure, immune suppression, chronic irritation, and chronic skin ulcers [4].
Furthermore, recent studies has indicated that CL may act as a risk factor for the development of various cancerous lesions on the skin, including BCC [5]. Hereby, a case of BCC developed on an active lesion of MCL, which had been partially managed at the Regional Leishmaniasis Control Center (RLCC), Yemen.
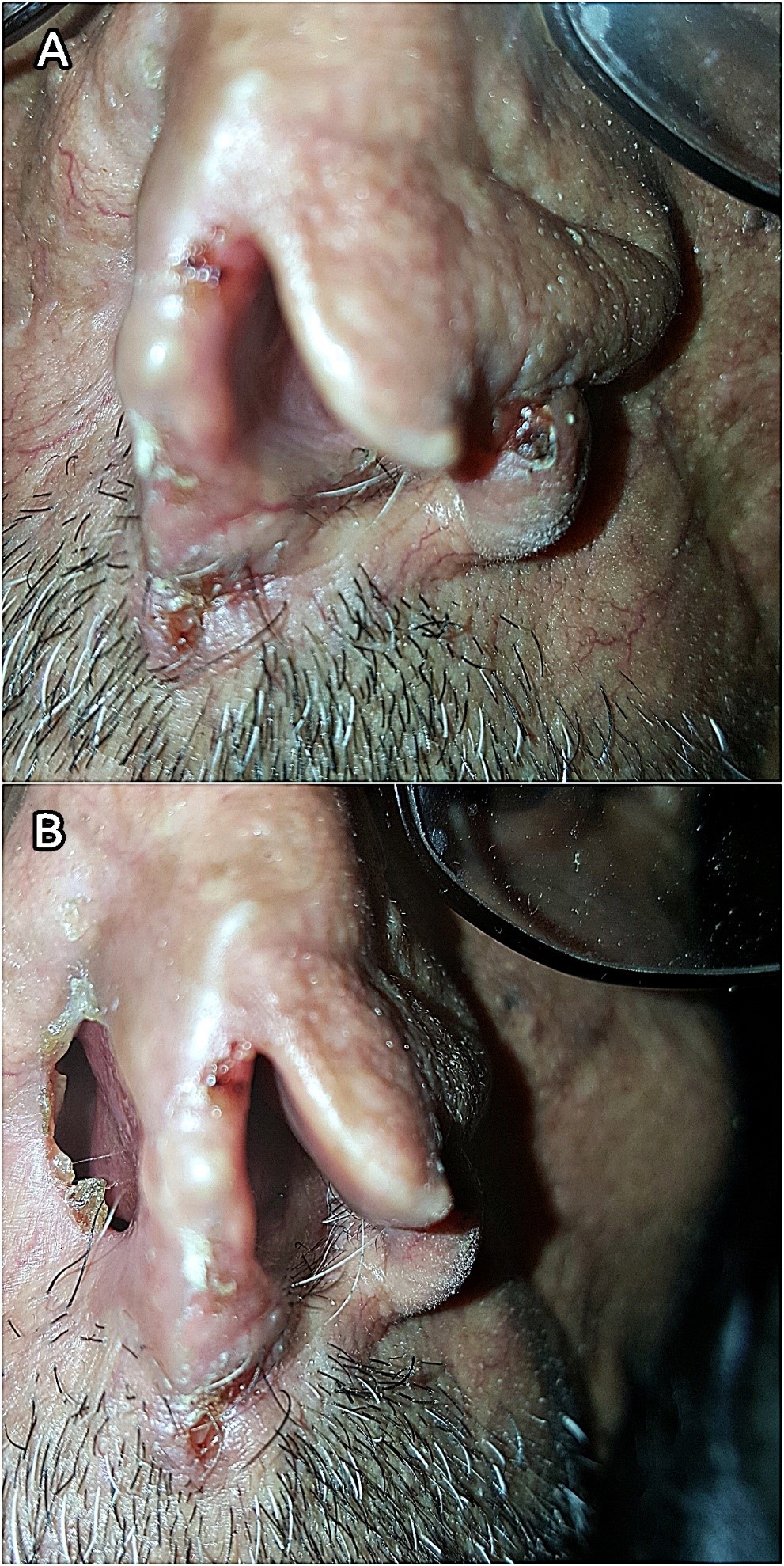
A 70-year-old male farmer from Raimah (a leishmaniasis endemic governorate at Central Yemen) visited RLCC clinic in Sana’a with three ulcerated lesions on his nose, the lesions was presented for at least seven months. Clinical examination revealed three scaling, shiny, ulcerated, red, and pearly nodules (5-10 mm in diameter) on the left alar rim (Figers. 1A and 1B).
History of the present illness revealed that over three years ago, the patient had an ulcer with diffuse erythema on the top of his nose, which was diagnosed by microscopic examination of a slit skin smear as MCL with a heavy infection of Leishmania parasites, and he was treated with intramuscular and intralesional injections of Sodium Stibogluconate (SSG). Although, it was responding well to SSG therapy, another concurrent pearly well-circumscribed ulcer with raised areas and ulcerated center started to develop at the site of the regressing leishmanial lesion.
Gradually it deeply invaded the nearby tissue and caused a significant destruction of the nose. BCC was diagnosed through histopathologic examination of a biopsied specimen. Accordingly, the cancer was excised by a plastic surgeon without post-operative radio- or chemotherapy. The patient was left with an unpleasant permanent disfigurement of the nose. (Figure 1B).
Seven months postoperative, three tumorous lesions had redeveloped on the left aral rim (Figers 1A and 1B), the lesions were biopsied and histopathologically identified as recurrent nodular BCC of the nose (Clark level IV); histopathologic study showed an ulcerated tumor growth composed of atypical basaloid cells arranged in cohesive nets with peripheral palisading and retraction artifact. Furthermore, the patient reported a history of prolonged sun exposure and a relevant family history of cutaneous leishmaniasis.
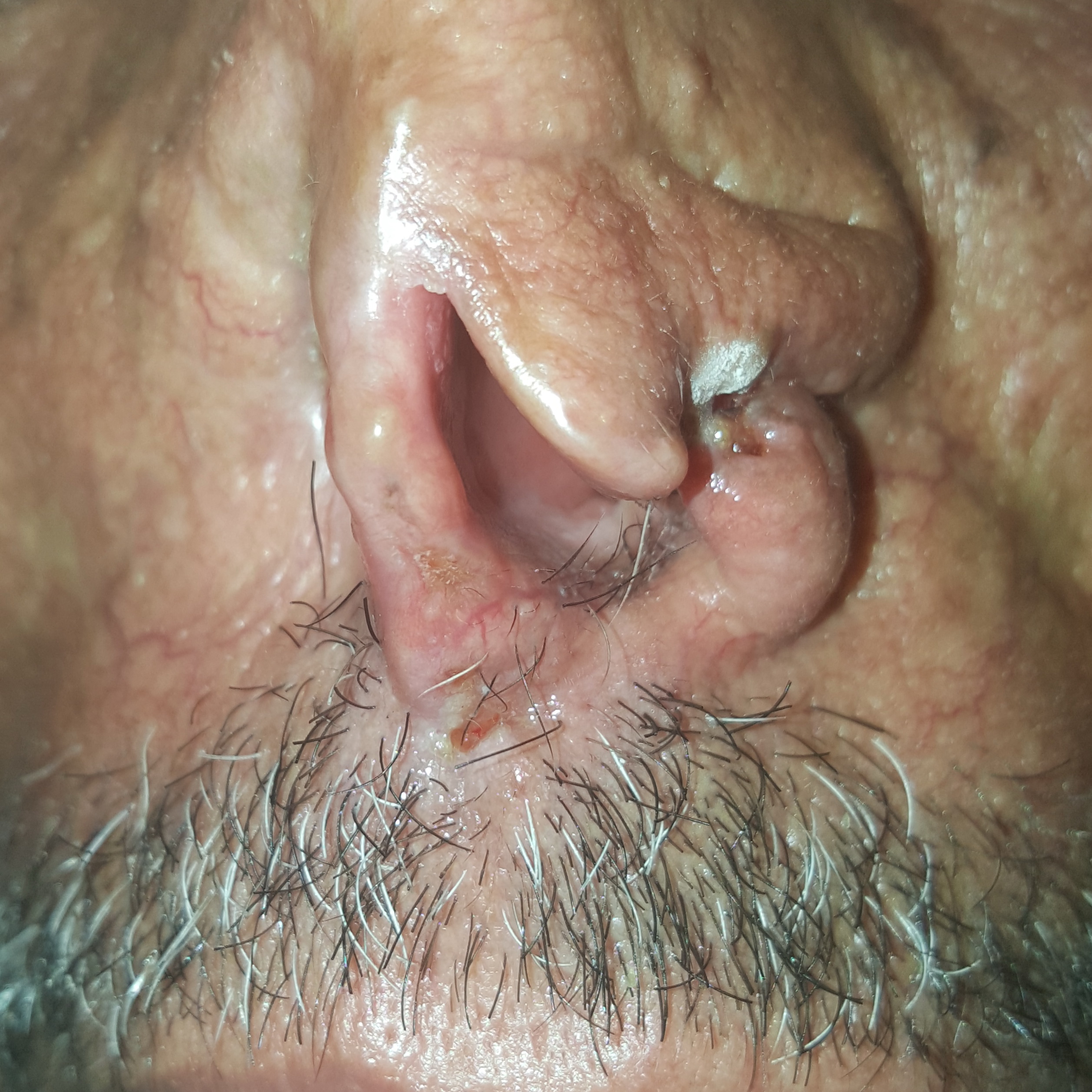
At RLCC, the patient had undergone two cryotherapy sessions with liquid nitrogen with two weeks interval (Figure 2). A complete clearance of the malignant lesions was obtained within one month of follow-up.
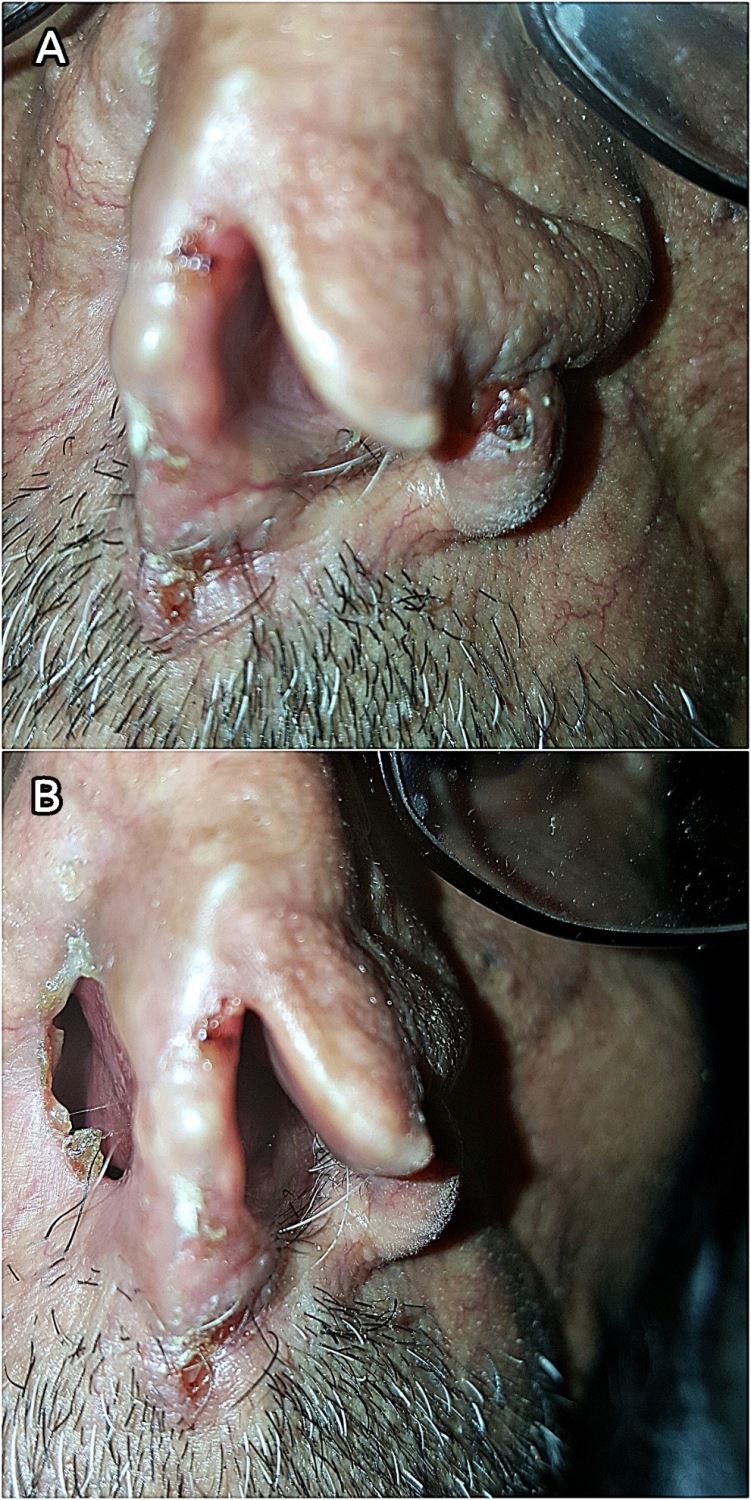
Figure 1. (A) Lesions on the rim of the left nostril (close view). (B) Post-operative disfigurement of the nose.
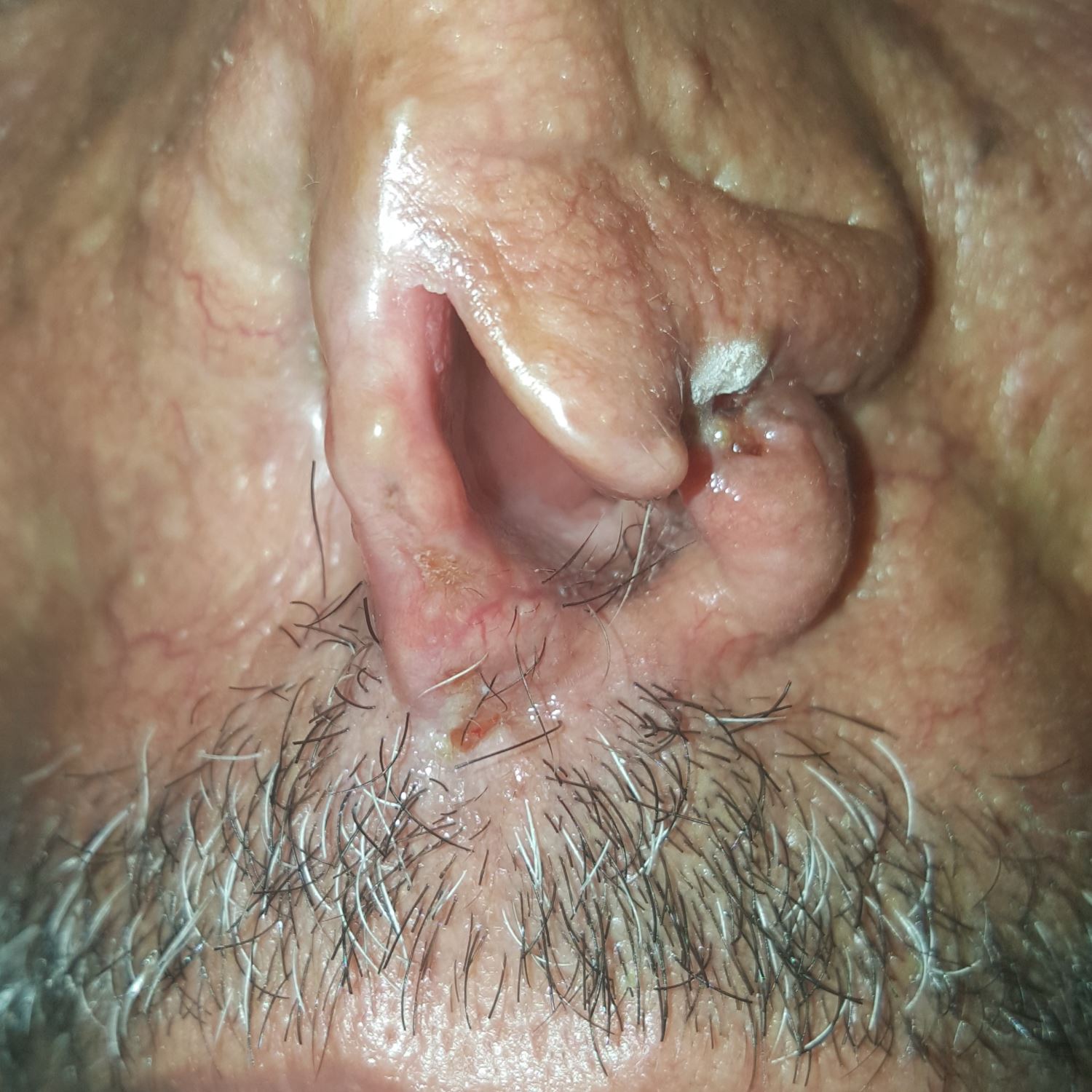
Figure 2. Cancerous lesions two weeks after the first cryotherapy session.
The extrinsic inflammation in cancers is caused by many factors, such as microbial infection, ultraviolet (UV) light radiation, autoimmune diseases, obesity, smoking, and irradiation, although by far the UV radiation is the most important factor in the malignant lesion development and progression. In contrast, cancer-intrinsic inflammation can be triggered by cancer-initiating mutations and can contribute to malignant progression through the recruitment and activation of inflammatory cells. Both extrinsic and intrinsic inflammation can result in immunosuppression, thereby providing a preferred background for tumor development [6,7].
Basal cell carcinoma, an epithelial malignant tumor with a low malignant potential, may arise in the setting of scars, draining sinuses, ulcers, burn sites, and foci of chronic inflammation. There is no unified and generally accepted classification of BCCs. When classifying BCCs, most authors start from the growth pattern, which gives more information about the bio-behavior and less often from the differentiation of tumors. All published works are in accord regarding their determination of the three basic groups of BCCs: nodular, superficial, and infiltrative, which present 80-90% of all BCCs. The diagnostic histological features common for all types of BCC are basaloid cells with a thin pale cytoplasm surrounding round or oval nuclei with a rough granulated chromatin pattern. The peripheral borderline cell layers are characterised by palisade arrangement and the surrounding stroma is often separated by artificially created slits, whereas the internal arrangement of the cells is rather chaotic. Most tumors originate in the epidermis and invade the dermis in the form of solid or cystic nodules or streaky projections creating various growth patterns [7,8].
In the literature, there are many cases of BCC developed during or after leishmaniasis on the skin [4,9]. The first article discussing CL as a possible predisposing factor for skin malignancy in Yemen was reported by Morsy et al. who described that the histopathological findings of a disfiguring CL lesion showed a picture as typical as of BCC [10].
In the previous studies, four possibilities were described to define the concurrent presence of BCC and CL in humans: (1) leishmaniasis masquerading as a malignant disorder; (2) leishmaniasis developing as a difficult to diagnose and treat infection among patients receiving chemotherapy for various malignant disorders; (3) simultaneous diagnosis of leishmaniasis and a neoplastic disorder in the same tissue samples of immunocompromised patients; and (4) direct involvement of Leishmania species in the pathogenesis of cancerous lesions [5].
Carcinogenic pathways in the current case of nodular, recurrent BCC (Clark level IV) of the nose may include, chronic inflammation induced by Leishmania parasites; chronic irritation induced by prolonged sun exposure (UV radiation); and subsequent dysfunction of the immune system [5,11] Further studies with appropriate methods and experimental approaches are recommended to carefully address these challenging hypotheses.
Received date: July 26, 2017
Accepted date: June 28, 2018
Published date: July 30, 2018
None
None
Written patient informed consent was obtained.
© 2018 The Author (s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY).
An increasing burden is loaded on clinical laboratories for daily testing of body fluids, including pleural fluid, peritoneal fluid (ascites), pericardial fluid, and cerebrospinal fluid. The request for detection of cellular components in the body fluid samples is usually delivered simultaneously to the hospital’s hematology laboratory and cytology laboratory. The medical technologists of hematology laboratory provide the screening result for suspicious malignant cells; meanwhile, the pathologists of cytology laboratory provide the presence of cancer cells at a diagnostic level. However, unfortunately, neither of these results reaches 100% sensitivity or specificity. To assess the clinical practice values, the present study performed a comparison of the hematology and cytology laboratories on the detection of malignancy in body fluids.
Patients with gynecological abdominal wall malignancies can benefit significantly from radical resection and autologous reconstruction. The pedicled anterolateral thigh flap is the preferred donor site, offering a reliable solution to abdominal wall reconstruction in this setting. The satisfactory results should prompt a more aggressive surgical approach for these patients. This article describes the authors' experiences with the abdominal reconstruction following surgical resection of gynecological abdominal wall malignancy using pedicled anterolateral thigh flap.
In Introduction it is stated “mucocutaneous leishmaniasis is the most prevalent form” whereas in literature we found the cutaneous form as most common.
ResponseYes, “mucocutaneous leishmaniasis is the most prevalent form and the single wet ulcer is the most common presentation of cutaneous leishmaniasis in Central Yemen” according to the cited reference number 1.
This case is most likely a happenstance occurring of two different pathologies at same/adjacent site. Both cutaneous form and basal cell carcinoma are common in the region and just concurrent appearance of two common ailments on same site (that too on mid-facial region and in elderly patient) makes it more likely a coincidence with no pathological relevance. Frequent occurrence of these type of lesions may alert towards role of cutaneous form as possible risk factor for basal cell carcinoma. On the basis of just one case it is not appropriate to label as risk factor.
ResponseThis type of association will stand as a hypothesis till clearly evidenced and was supported by numerous reports which had been listed in the reference section. According to your kind observations, the manuscript had been justified and revised, 2 references were added as well.
As I mentioned previously the histopathologic slide should be added to the article instead of its report.
ResponseA paragraph of the histopathologic features of BCC was added to Discussion section. We don't have the histopathologic slide since I can't reach the patient who lives in a remote area.
I just have reviewed the revised version and I am satisfied with the modification and the reply from the author. In present form this article can be considered fit for publication in the category of low to moderate priority for publication.
ResponseThank you very much.
I reviewed the modifications; they are all fine. I still notice some small grammar mistakes (such as in Leishmania major is referred to as the principal causative agent in these cases), but I am sure they will be corrected during the Editing process. The manuscript is suitable for publication after these modifications.
ResponseThe grammar errors will be corrected, thank you very much.
Al-Kamel MA. Basal cell carcinoma developed on an active lesion of mucocutaneous leishmaniasis: A case report from Yemen. Arch Clin Dermatol 2018;1(1):4. https://doi.org/10.24983/scitemed.acd.2018.00072